肝星状细胞和库普弗细胞之间的细胞外囊泡依赖性串扰促进了它们的相互激活
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-05-30
DOI:10.1016/j.bbadis.2025.167914
引用次数: 0
摘要
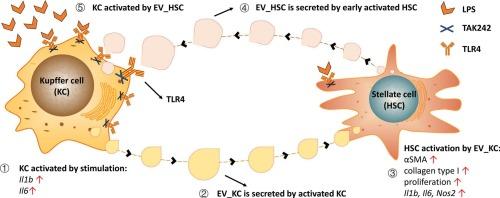
背景,目的肝纤维化是由慢性炎症驱动的肝星状细胞(HSC)活化和过度的细胞外基质(ECM)沉积引起的。库普弗细胞(KCs)在HSC激活中起着核心作用。我们之前的研究表明,hsc分泌的因子,特别是细胞外囊泡(EVs),可以激活KCs。然而,活化的KCs对hsc的相互作用仍然知之甚少。本研究探讨了HSC和KCs之间的双向串扰,重点研究了kc来源的EVs在调节HSC激活和纤维化进展中的作用。方法从雄性Wistar大鼠中分离原代hsc和KCs。造血干细胞与KCs共培养24小时,以评估炎症和激活标志物。第1天给予lps刺激的kc来源的ev和对照组hsc。利用LPS和toll样受体4 (TLR4)抑制剂TAK-242详细研究细胞间通讯。结果sco培养的HSC和KCs表现出相互激活,两种细胞类型的炎症标志物升高,HSC促纤维化活性增强。促炎(LPS)激活的KCs以tlr4依赖的方式放大HSC的激活。这种增强的HSC激活部分归因于电动汽车。结论在共培养中,KCs和hsc以依赖tlr4的方式相互激活。这种双向激活被促炎介质增强。kc来源的EVs(部分)激活hsc,这可能有助于体内肝纤维化的进展。通过阻断TLR4信号传导调节KC激活,可能会改变EV分泌或货物组成,减少HSC激活和纤维化进展。靶向这种ev介导的串扰可能为肝纤维化提供新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Extracellular vesicle-dependent crosstalk between hepatic stellate cells and Kupffer cells promotes their mutual activation
Background & aims
Hepatic fibrosis results from hepatic stellate cell (HSC) activation and excessive extracellular matrix (ECM) deposition, driven by chronic inflammation. Kupffer cells (KCs) play a central role in HSC activation. We previously showed that HSC-secreted factors, particularly extracellular vesicles (EVs), activate KCs. However, the reciprocal effects of activated KCs on HSCs remain poorly understood. This study investigates the bidirectional crosstalk between HSCs and KCs, focusing on the role of KC-derived EVs in regulating HSC activation and fibrosis progression.
Methods
Primary HSCs and KCs were isolated from male Wistar rats. HSCs were co-cultured with KCs for 24 h to assess inflammatory and activation markers. LPS-stimulated KC-derived EVs and controls were administered to HSCs on day 1. LPS and the Toll-like receptor 4 (TLR4) inhibitor TAK-242 were used to investigate the intercellular communication in detail.
Results
Co-cultured HSCs and KCs showed mutual activation, demonstrated by elevated inflammatory markers in both cell types and enhanced HSC pro-fibrotic activation. Pro-inflammatory (LPS)-activated KCs amplified HSC activation in a TLR4-dependent fashion. Part of this augmented HSC activation was attributed to EVs.
Conclusions
In co-culture, KCs and HSCs show mutual activation in a TLR4-dependent fashion. This bidirectional activation is augmented by pro-inflammatory mediators. KC-derived EVs (partially) activate HSCs, which might contribute to progression of liver fibrosis in vivo. Modulating KC activation, such as by blocking TLR4 signaling, may alter EV secretion or cargo composition, reducing HSC activation and fibrosis progression. Targeting this EV-mediated crosstalk could provide novel therapeutic strategies for liver fibrosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


