通过自由基-阴离子接力铁催化烯烃和炔的烷基化氨基磺化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究了以Na2S2O4为还原剂和砜源,铁催化烯烃和炔与烷基卤化物和O-Ts活化的羟胺的烷基化氨基磺化反应。发现金属-电子穿梭催化对于不需要有机金属中间体而高效生成磺酰基自由基和阴离子至关重要。这种方法可以从容易获得的烯烃和Na2S2O4中高效地获得磺胺,并且具有广泛的底物范围。本文章由计算机程序翻译,如有差异,请以英文原文为准。
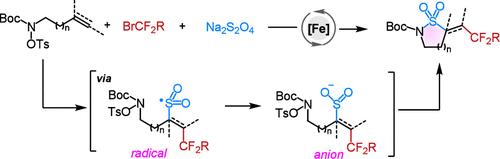
Iron-Catalyzed Alkylative Aminosulfonylation of Alkenes and Alkynes via Radical-Anion Relay
A novel Fe-catalyzed alkylative aminosulfonylation of alkenes and alkynes with alkyl halides and O-Ts activated hydroxylamines by using Na2S2O4 as a reductant and sulfone source has been developed. The metal-electron-shuttle catalysis was discovered to be vital for the highly efficient generation of sulfonyl radicals and anions without requiring organometallic intermediates. This method provides efficient access to sulfonamides from easily available alkenes and Na2S2O4 and features a broad substrate scope.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


