镍催化C-N键活化吡啶的还原烷基化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在本研究中,我们以2-吡啶基吡啶酮为底物,通过镍催化的交叉亲电偶联平台,通过C-N键活化与烷基溴进行还原转化,在室温下高效地构建了2-烷基吡啶。该反应允许在两种偶联剂上使用各种敏感的电子取代基。使用多种吡啶基吡啶酮作为吡啶基前体,产率可达95%。此外,在天然产物和药物后期功能化中的应用也增强了它的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
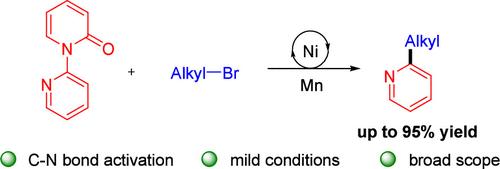
Nickel-Catalyzed Reductive Alkylation of Pyridines via C–N Bond Activation
In this work, we utilized 2-pyridylpyridones as substrates for a reductive transformation with alkyl bromides via C–N bond activation through a Ni-catalyzed cross-electrophile coupling platform to efficiently construct 2-alkylpyridines at room temperature. The reaction allowed the use of a variety of sensitive electronic substituents on both coupling agents. Yields up to 95% can be achieved using a wide array of pyridylpyridones as pyridyl precursors. In addition, applications in the late-stage functionalization of natural products and drugs enhanced its potential.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


