揭示设计的芳基糖苷对牛碱性磷酸酶葡萄糖苷酶的混交活性
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
新催化功能的自然进化归因于酶中潜在(混杂)活性的存在。在此,我们提出了碱性磷酸酶的催化混杂性的第一个例子,从初级活性位点可以催化芳基α-糖苷的水解,除了它们的底物混杂性。为了探究其结构特征,我们合成了多种芳基糖苷,结果表明,只有一类特定的α-糖苷在芳基上有一个游离的邻近羟基。因此,我们的发现对酶功能的进化和隐藏的多样性具有启示意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
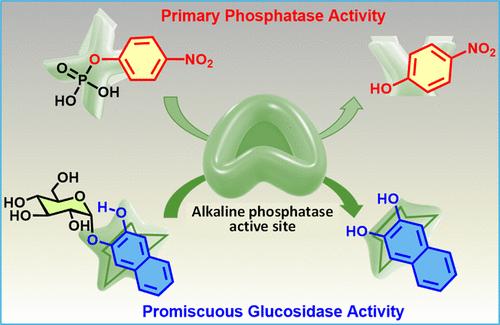
Unveiling the Promiscuous Glucosidase Activity of Bovine Alkaline Phosphatase with Designed Aryl Glycosides
The natural evolution of new catalytic functions is attributed to the existence of latent (promiscuous) activities in enzymes. Herein, we present the first example of catalytic promiscuity of alkaline phosphatase from the primary active site that can catalyze the hydrolysis of aryl α-glucosides, in addition to their well-explored substrate promiscuity. To explore structural features, various aryl glycosides were synthesized, which showed the hydrolysis of only a specific class of α-glucosides with a free neighboring hydroxyl group on the aryl moiety. Thus, our discovery has implications for the evolution of enzyme functions and hidden diversity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


