RuO4和NOX在RuO2表面竞争吸附的从头算研究:一起严重核事故中RuO4释放的评估
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
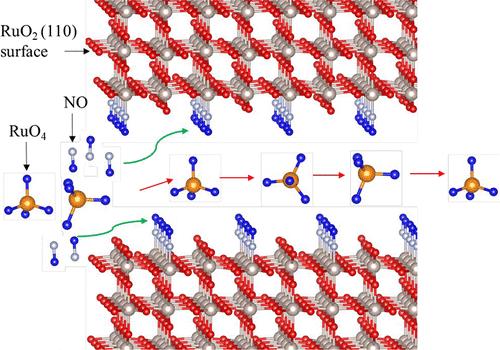
在当前能源和地缘政治危机的背景下,核安全最近已成为一个中心问题。准确估计核设施可能释放的放射性物质的性质和程度,对于评估和优化安全计划至关重要。计算技术似乎是一种强大的工具来了解这些物种的形成和运输,并量化它们的数量。本研究的重点是气态四氧化钌(RuO4)的分解,这是一种在燃料后处理工厂发生事故时可能释放到应急通风管道中的裂变产物。RuO4(g)在有效表面的运输过程中分解成固体RuO2 (c)和无定形O2(g)。然而,环境中存在的其他物质,如氮氧化物,可能与生长中的沉积物(RuO2)表面相互作用,毒害反应位点-à-vis,使RuO4吸附/分解,有利于其以气相运输到环境中。为了理解这种对RuO2表面的中毒效应,我们在PBE水平上进行了周期性密度泛函理论计算。色散校正(PBE+D级)的影响在辅助信息中给出。在RuO2(110)表面计算不同覆盖率的RuO4、NO和NO2分子。我们提供了吸附吉布斯自由能、等温线和事故条件下的中毒效应。我们的研究结果表明,NOX似乎对RuO2(110)表面有明显的中毒影响,减少了RuO4(g)的吸附,这是冷凝机制的第一步。我们的计算表明,这种影响随着NOX分压的增加而增加,随着温度的升高而降低。这项研究的结果也将在其他领域引起人们的兴趣,比如涉及RuOx表面的多相催化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ab Initio Investigation of the Competitive Adsorption of RuO4 and NOX on a RuO2 Surface: Assessment of RuO4 Release in a Severe Nuclear Accident
Nuclear safety has recently become a central issue in the context of the current energy and geopolitical crises. Accurate estimates of the nature and extent of radioactive species potentially released from nuclear facilities are essential for assessing and optimizing safety plans. Computational techniques appear as a powerful tool to understand the formation and transport of such species and to quantify their amount. This study focuses on the decomposition of gaseous ruthenium tetroxide (RuO4), a fission product likely to be released into the emergency ventilation ducts in the event of an accident at a fuel reprocessing plant. RuO4(g) is expected to decompose during its transport on the available surfaces into solid RuO2 (c) and amorphous O2(g). However, other species like NOX present in the environment might interact with the surfaces of the growing deposit (RuO2), poisoning the reactive sites vis-à-vis the RuO4 adsorption/decomposition and favoring its transport in the gas phase to the environment. To understand this poisoning effect on RuO2 surfaces, we carried out periodic density functional theory calculations at the PBE level. The impact of dispersion corrections (PBE+D level) is presented in the Supporting Information. RuO4, NO, and NO2 molecules at different coverages are computed on the RuO2 (110) surface. We provide adsorption Gibbs free energies, isotherms, and the poisoning effect in the conditions of an accident. Our results show that the NOX seems to have a noticeable poisoning impact on the RuO2 (110) surface, reducing the adsorption of RuO4(g), the first step of the condensation mechanism. Our calculations show that this impact increases with NOX partial pressure and decreases with temperature. The results of the study will also be of interest in other fields like heterogeneous catalysis, where RuOx surfaces are involved.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


