十钨酸盐催化乙烯磺酰氟的光化学反应:C-H功能化的关键试剂
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文描述了在十钨酸四丁基铵催化的C-H键功能化反应中,乙烯磺酰氟(ESF)作为一种强有力的关键试剂的使用。ESF作为一种强效的Michael受体,可与光催化剂抽离氢原子产生的自由基反应,生成多种磺酰氟化合物。这些磺酰氟作为多功能中间体,通过分子间和分子内SuFEx反应合成磺胺、磺胺和磺酸盐。此外,该方法还包括一种新的由醛直接合成β功能化酮的一锅法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
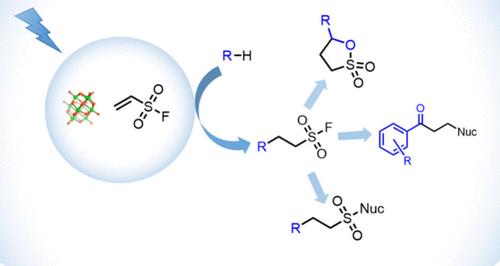
Decatungstate-Catalyzed Photochemical Reactions of Ethenesulfonyl Fluoride: A Linchpin Reagent for C–H Functionalization
This work describes the use of ethenesulfonyl fluoride (ESF) as a powerful linchpin reagent in tetrabutylammonium decatungstate-catalyzed C–H bond functionalization reactions. As a potent Michael acceptor, ESF can react with radicals generated by hydrogen atom abstraction by the photocatalyst to produce a variety of sulfonyl fluoride compounds. These sulfonyl fluorides serve as versatile intermediates for the synthesis of sultones, sulfonamides, and sulfonates via inter- and intramolecular SuFEx reactions. In addition, this method includes a novel one-pot synthesis of β-functionalized ketones directly from aldehydes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


