CD56在肿瘤内NK细胞中的作用:协调NK细胞介导的膀胱癌抗肿瘤作用
IF 7.7
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
摘要
膀胱癌(BCa)对免疫治疗表现出良好的反应,但由于肿瘤免疫环境不充分,很大比例的患者未能表现出反应。我们之前的研究表明NK细胞是BCa中主要的肿瘤浸润淋巴细胞之一,并与患者生存率的提高有关。然而,这种联系仅在CD56bright NK细胞中观察到,而CD56dim亚群在BCa晚期表现出降低的细胞毒性和更高的积累。然而,CD56在BCa中NK细胞功能中的作用尚不清楚。通过流式细胞术和细胞毒性实验,我们发现CD56缺失后,NK92细胞对BCa的细胞毒性和活性显著降低。此外,迁移实验和原子力显微镜显示,CD56缺失损害了NK92细胞对膀胱肿瘤细胞的迁移和粘附,减少了NK92细胞介导的BCa细胞凋亡。长期暴露于膀胱肿瘤导致NK92细胞中CD56丢失,提示肿瘤通过CD56减少诱导NK92细胞功能障碍,这与我们之前的研究结果一致。共聚焦显微镜显示CD56和磷酸化的Pyk2重叠,Pyk2是肿瘤免疫突触的一个关键激酶,可能介导下游的细胞毒性作用。阻断Pyk2磷酸化可降低cd56介导的NK92细胞活化,降低NK92细胞介导的对BCa的细胞毒性。最后,我们发现CD56也在BCa细胞中表达,并且可能是基于NK细胞的免疫治疗的预测性生物标志物,其脱落表明NK细胞逃避的机制。我们的研究在BCa中发现了一个新的先天免疫轴,从而更好地理解肿瘤内NK细胞生物学和推进NK细胞靶向治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
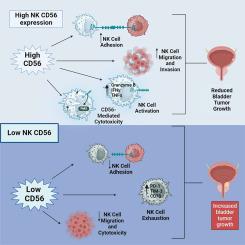
CD56 on intratumoral NK cells: orchestrating NK cell-mediated anti-tumor effects in bladder cancer
Bladder cancer (BCa) exhibits favorable responses to immunotherapy, but a significant percentage of patients fail to show a response owing to an inadequate tumor-immune landscape. We previously showed that NK cells are one of the predominant tumor-infiltrating lymphocytes in BCa and correlate with improved patient survival. However, that link was observed only with CD56bright NK cells while the CD56dim subset exhibited reduced cytotoxicity and higher accumulation in advanced BCa stages. The role of CD56 in NK cell functionality in BCa, however, remains unclear. Using flow cytometry and cytotoxicity assays, we demonstrated a significant decrease in cytotoxicity and activation of NK92 cells against BCa upon CD56 deletion. Further, migration assays and atomic force microscopy showed CD56 deletion impaired NK92 cell migration and adhesion to bladder tumor cells, reducing NK92 cell-mediated apoptosis of BCa cells. Prolonged exposure to bladder tumors led to CD56 loss in NK92 cells, suggesting tumor-induced NK92 cell dysfunction via CD56 reduction, consistent with our previous findings. Confocal microscopy revealed an overlap of CD56 and phosphorylated Pyk2, a critical kinase at the tumor-immune synapse, potentially mediating the downstream cytotoxicity effects. Blocking Pyk2 phosphorylation decreased CD56-mediated NK92 cell activation and reduced NK92 cell-mediated cytotoxicity against BCa. Finally, we showed that CD56 is also expressed by BCa cells and may be a predictive biomarker for NK cell-based immunotherapy, with its shedding indicating a mechanism for NK cell evasion. Our study identifies a novel innate-immune axis in BCa, leading to a better understanding of intratumoral NK cell biology and advancing NK cell-targeted treatments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


