z -方案氧化钨铜光催化水分解
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
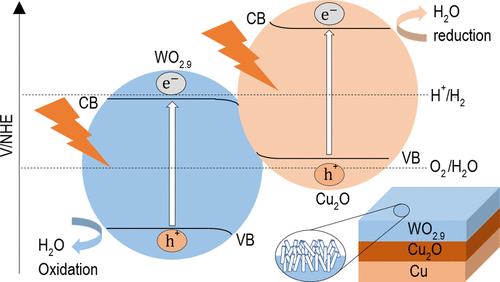
能源技术的一个重大限制是大规模能源储存的挑战,这阻碍了可再生能源的广泛采用。要从目前对化石燃料发电的依赖(这是高度可预测的)转变过来,必须有一个具有成本效益的大规模能源储存解决方案。一种很有前途的技术是直接利用阳光将水分解成氢和氧,这被称为光电化学(PEC)水分解。这种环保的方法在生产可再生氢方面备受关注。将太阳能转化为氢的PEC水分解电池的效率在很大程度上取决于所使用的半导体材料,这表明它们是生产绿色氢的有前途的技术。我们开发了一种用于水分解的PEC电池,它利用阳光驱动化学反应,将水分子分离成氢气和氧气。这种方法可以将氢气储存起来,并作为一种可靠的能源使用,类似于化石燃料,但不会产生温室气体。采用热丝化学气相沉积(HWCVD)方法在氧化铜衬底上制备了WO2.9的纳米异质结构。所得WO2.9/Cu2O/Cu纳米结构为棒状,平均直径为50±8 nm。该光催化剂在可见光下表现出优异的产氢活性,在没有任何施加偏压的情况下,太阳能制氢(STH)效率约为1%。这项研究强调了创造高性能、低成本的绿色制氢光催化剂的有希望的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Z-Scheme Tungsten Copper Oxide for Photocatalytic Water Splitting
A significant limitation in energy technology is the challenge of large-scale energy storage, which hinders the widespread adoption of renewable energy sources. To transition from the current reliance on fossil-fuel-generated power, which is highly predictable, there must be a cost-effective solution for large-scale energy storage. One promising technology is the direct use of sunlight to split water into hydrogen and oxygen, which is known as photoelectrochemical (PEC) water splitting. This eco-friendly method has garnered significant attention for producing renewable hydrogen. The efficiency of PEC water-splitting cells, which convert solar energy to hydrogen, largely depends on the semiconductor materials used, demonstrating that they are a promising technology for the production of green hydrogen. We developed a PEC cell for water splitting that utilizes sunlight to drive the chemical reaction, separating water molecules into hydrogen and oxygen gas. This approach allows hydrogen gas to be stored and used as a reliable power source, similar to fossil fuels but without generating greenhouse gases. We have prepared a nanostructured heterostructure of WO2.9 using the hot wire chemical vapor deposition (HWCVD) method on an oxidized copper substrate. The resulting WO2.9/Cu2O/Cu nanostructures are rod-shaped with an average diameter of 50 ± 8 nm. The photocatalyst demonstrates excellent hydrogen production activity under visible light, achieving a solar-to-hydrogen (STH) efficiency of approximately 1% without any applied bias potential. This study highlights a promising pathway for creating high-performance, low-cost photocatalysts for green hydrogen production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


