烯的光诱导1,2-碳亚砜化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了一种温和、高效的光催化方案,以n -羟基邻苯二胺酯为碳源,烷基硫代硫酸盐为硫源,进行1,2-碳磺酰化反应。这种操作简单的方法为合成二基硫醚,包括甲基硫醚衍生物提供了新的途径。该反应具有广泛的底物范围和优异的官能团耐受性。机理研究已经阐明了反应机理,并证实了烷基自由基优先参与反应,使烯烃产生高度区域选择性的1,2-碳亚砜化反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
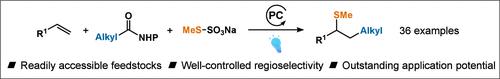
Photoinduced 1,2-Carbosulfenylation of Alkenes
Herein, we report a mild, efficient photocatalytic protocol for 1,2-carbosulfenylation of alkenes using N-hydroxyphthalimide esters as carbon sources and alkyl thiosulfate salts as sulfur sources. This operationally simple methodology provides a novel avenue for synthesizing dialkylthioether, including methylthioether, derivatives. The reaction has a broad substrate scope and excellent functional group tolerance. Mechanistic studies have elucidated the mechanism and confirmed the preferential participation of alkyl radicals in the reaction, producing the highly regioselective 1,2-carbosulfenylation of alkenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


