非经典碳正离子催化纯烯丙基叔醇的SN1反应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们提出了一种通过非经典环丙基碳正离子中间体形成的同丙基叔醇的立体保持性亲核取代。这种策略可以创建高度拥挤的三级中心,并保留立体控制,解决SN1机制中碳正离子不稳定性和反应性的典型挑战。CPC中间体的稳定对于实现精确的区域选择性和立体选择性至关重要,显著提高了sn1型机制在复杂分子合成中的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
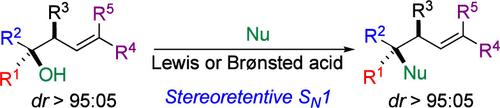
Formally Stereoretentive SN1 Reactions of Homoallylic Tertiary Alcohols Via Nonclassical Carbocation
We present a stereoretentive nucleophilic substitution of homoallylic tertiary alcohols via the formation of a nonclassical cyclopropyl carbinyl (CPC) carbocation intermediate. This strategy enables the creation of highly congested tertiary centers with preserved stereocontrol, addressing the typical challenges of carbocation instability and reactivity in SN1 mechanisms. The stabilization of the CPC intermediate is crucial for achieving precise regio- and stereoselectivity, significantly enhancing the utility of SN1-type mechanisms in complex molecule synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


