二甲基丙烯基焦磷酸(DMAPP)的稳定同位素标记揭示了枯草芽孢杆菌产孢过程中类异戊二烯生物合成的区室化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
类异戊二烯是两种五碳(C5)异构体,即焦磷酸异戊烯基(IPP)和焦磷酸二甲基烯丙基(DMAPP)的重要代谢物。尽管这些异构体是类异戊二烯生物合成的核心底物,但由于缺乏可用的化学工具,很难追踪它们在下游代谢物中的独立结合。为了解决这个问题,我们开发了一种细胞渗透的、稳定的同位素标记的DMAPP类似物,它使用自焚酯来掩盖β-磷酸盐,允许有效的细胞摄取,并直接对枯草芽孢杆菌中DMAPP的下游产物进行同位素标记。我们证明了13c标记的、酯保护的DMAPP (13C3 SIE-DMAPP)在不抑制内源性IPP和DMAPP产生的情况下,与甲基萘醌-7 (MK-7)显著结合。通过敲除异戊烯基焦磷酸异构酶(IPPI)的表达,我们在MK-7的DMAPP衍生位置获得了特定的13C同位素标记,并证明IPPI过表达使枯草芽孢杆菌利用DMAPP作为其唯一的类异戊烯来源。最后,我们追踪了DMAPP在枯草芽孢杆菌从营养生长到孢子形成过程中的结合,揭示了室特异性同位素标记模式,强调了母细胞和孢子内类异戊二烯代谢在孢子形成过程中的代谢独立性。这种稳定同位素标记的DMAPP的引入有助于在天然生物环境中追踪DMAPP衍生的代谢物,并为研究戊烯基代谢、萜烯生物合成途径和细胞类异戊烯池的调节机制开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
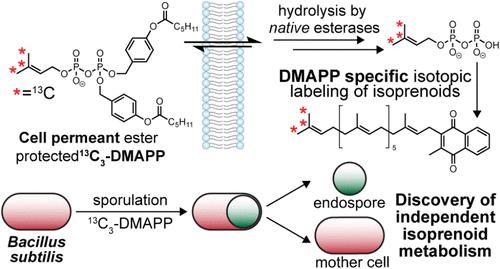
Stable Isotopic Labeling of Dimethylallyl Pyrophosphate (DMAPP) Reveals Compartmentalization of Isoprenoid Biosynthesis during Sporulation in Bacillus subtilis
Isoprenoids are essential metabolites whose biosynthesis originates from two five-carbon (C5) isomers, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). Although these isomers serve as the core substrates for isoprenoid biosynthesis, tracking their independent incorporation into downstream metabolites is difficult due to the lack of available chemical tools. To address this issue, we have developed a cell-permeant, stable isotope-labeled analog of DMAPP that employs self-immolating esters to mask the β-phosphate, allowing for efficient cellular uptake and direct forward isotopic labeling of downstream products of DMAPP in Bacillus subtilis. We demonstrate that 13C-labeled, ester-protected DMAPP (13C3 SIE-DMAPP) achieves significant incorporation into menaquinone-7 (MK-7), regardless of inhibition of endogenous production of IPP and DMAPP. By knocking out isopentenyl pyrophosphate isomerase (IPPI) expression, we achieve specific 13C isotopic labeling exclusively at DMAPP-derived positions of MK-7 and demonstrate that IPPI overexpression enables B. subtilis to utilize DMAPP as its sole isoprenoid source. Finally, we tracked DMAPP incorporation during the transition from vegetative growth to sporulation, revealing compartment-specific isotope labeling patterns that underscore the metabolic independence of isoprenoid metabolism in the mother cell and endospore during sporulation in B. subtilis. The introduction of this stable isotope-labeled DMAPP facilitates the tracking of DMAPP-derived metabolites in native biological contexts and opens new avenues for studying prenyl metabolism, terpene biosynthetic pathways, and the regulatory mechanisms governing cellular isoprenoid pools.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


