TRPM8蛋白动力学与配体结构和细胞功能相关
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
蛋白质动力学已成为与各种系统功能相关的关键特征。在这里,基于核磁共振的研究结合计算化学信息学和细胞功能来确定人类感冒和薄荷醇受体TRPM8动力学、化学结构和细胞效力之间的关系。TRPM8是多种疼痛适应症的有效靶标,但通常在临床上受到靶标副作用的限制,影响热感和体温调节。本研究表明,调节小分子配体文库的TRPM8的化学信息学分析与细胞功能相关。电生理学研究进一步证实了这一关系,并显示了化学结构与化合物效价等功能特征之间的相关性。TRPM8电压传感样结构域(包含典型薄荷醇配体结合位点)的溶液核磁共振研究表明,配体结合构象选择了核磁共振检测到的TRPM8动力学,其定量与化学结构相关。化学结构和蛋白质动力学之间的关系可以预测性地使用,其中化学结构可以预测潜在降维空间中的动力学。此外,动态系综构象选择的稳健性通过不同的相关和不同的化学型、信噪灵敏度和样本偏差来评估。综上所述,本研究确定了蛋白质动力学可以作为化学结构和细胞功能之间的可量化桥梁,这对在困难系统中发现药物具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
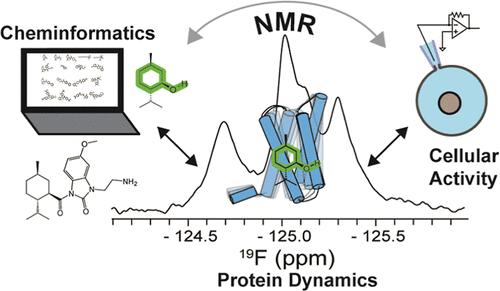
TRPM8 Protein Dynamics Correlates with Ligand Structure and Cellular Function
Protein dynamics has emerged as a key feature associated with function in various systems. Here, NMR-based studies coupled with computational cheminformatics and cellular function are leveraged to identify a relationship between human cold and menthol receptor TRPM8 dynamics, chemical structure, and cellular potency. TRPM8 is a validated target for a variety of pain indications but generally has been clinically limited by on-target side effects, impacting thermosensing and thermoregulation. This study shows that cheminformatic analysis of a TRPM8 regulating small-molecule ligand library correlates with cellular function. Electrophysiology studies further validate the relationship and show a correlation between the chemical structure and functional features such as compound potency. Solution NMR studies of the TRPM8 voltage sensing-like domain, which houses the canonical menthol ligand binding site, show that ligand binding conformationally selects NMR-detected TRPM8 dynamics in a manner that quantitatively correlates with the chemical structure. The relationship between chemical structure and protein dynamics can be used predictively, where a chemical structure is predictive of dynamics in a latent reduced dimensionality space. Moreover, the robustness of the conformational selection of the dynamic ensemble is evaluated by varying related and divergent chemotypes, signal-to-noise sensitivity, and sample bias. Taken together, this study identifies that protein dynamics can serve as a quantifiable bridge between the chemical structure and cellular function, which has implications for drug discovery in difficult systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


