具有羟基喹啉壳的十核Ni(II)羰基簇的可调自组装:具有可逆溶剂/温度驱动相变和选择性气体分离的稳健多孔网络
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
利用分子金属团簇作为非共价多孔材料(npm)的构建单元,将有机和无机亚基的多功能功能与非共价相互作用控制的分子固体的柔软性和柔韧性相结合,是一种很有前途的策略。然而,基于非共价力驱动的自组装的健壮多孔功能框架的开发仍然具有很高的挑战性。在这里,我们报道了一个离散的十核Ni(II)羟基喹啉-羰基簇[Ni10(μ6-CO3)4(L)12]的合成和表征,根据结晶条件,它可以自组装成两种微孔框架:金刚石类WUT-1(Ni)和黄铁矿类WUT-2(Ni)。两种多晶型之间的转变也可以通过温度或暴露于特定有机溶剂的蒸气而选择性地触发,这伴随着材料从非晶相中容易恢复结晶度。此外,这两种材料对各种化学环境都表现出出色的稳健性,包括空气/水分和水的稳定性,并表现出有趣的气体吸附特性。值得注意的是,与之前报道的同结构Zn(II)类似物相比,WUT-1(Ni)表现出显著的气体吸收增强,代表了npm中最高的H2吸收之一。反过来,超微孔WUT-2(Ni)框架的紧密空隙促进了与气体分子的选择性相互作用,从而使CO2在CH4和N2上的吸附具有出色的选择性。本文的研究表明,金属中心的性质对等结构纳米团簇的自组装以及由此产生的微孔框架的性质具有深远的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
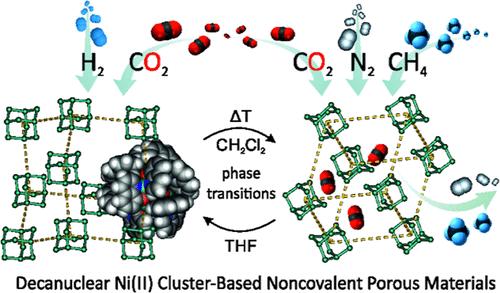
Tunable Self-Assembly of Decanuclear Ni(II) Carbonato Clusters with a Hydroxyquinolinato Shell: Robust Porous Networks with Reversible Solvent-/Temperature-Driven Phase Transitions and Selective Gas Separation
The utilization of molecular metal clusters as building units of noncovalent porous materials (NPMs) is a promising strategy, combining the versatile functionality of organic and inorganic subunits with the softness and flexibility of molecular solids controlled by noncovalent interactions. However, the development of robust porous functional frameworks based on self-assembly driven by noncovalent forces is still highly challenging. Herein, we report the synthesis and characterization of a discrete decanuclear Ni(II) hydroxyquinolinato-carbonato cluster, [Ni10(μ6-CO3)4(L)12], which, depending on the crystallization conditions, self-assembles into either of two microporous frameworks: diamondoid WUT-1(Ni) and pyrite WUT-2(Ni). The transitions between both polymorphs can also be selectively triggered by temperature or exposure to vapors of a particular organic solvent, which is accompanied by the easy recovery of crystallinity by the materials from the noncrystalline phase. Moreover, both materials show excellent robustness toward various chemical environments, including air/moisture and water stability, and demonstrate interesting gas adsorption properties. Remarkably, WUT-1(Ni) exhibits significant enhancement in gas uptake compared to the previously reported isostructural Zn(II) analogue, WUT-1(Zn), representing one of the highest H2 uptakes among NPMs. In turn, tighter voids of the ultramicroporous WUT-2(Ni) framework facilitate selective interactions with gas molecules, resulting in outstanding selectivity in the adsorption of CO2 over CH4 and N2. The presented studies demonstrate the profound role of the character of metal centers on the self-assembly of isostructural nanoclusters as well as properties of the resulting microporous frameworks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


