基于共价有机框架的人工金属抗氧化酶的空间构型引导设计,用于抑制炎症级联和调节骨稳态
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
骨组织中强烈的氧化应激可触发中性粒细胞的过度活化,从而引起炎症级联反应,使骨稳态恶化。在此,受天然抗氧化酶催化中心的启发,我们介绍了基于共价有机框架(COF)的人工金属抗氧化酶的空间构型引导设计,以抑制炎症级联反应和调节骨稳态。具体来说,钌配位的六胺氧六氮杂萘COF (S-HACOF-Ru)具有空间构型的富电子中心,具有出色的抗氧化酶类活性氧(ROS)清除能力,可有效减轻氧化应激。因此,S-HACOF-Ru有效地阻止中性粒细胞胞外陷阱的产生并抑制髓过氧化物酶(MPO)的释放。S-HACOF-Ru通过阻止mpo诱导的核因子κ B活化和抑制促炎巨噬细胞极化,成功阻断了中性粒细胞-巨噬细胞的炎症级联反应。这种干预通过从骨吸收到组织再生的转变来促进骨稳态,这可以有效地抑制牙周组织的牙槽骨丢失和逆转踝关节腔的软骨损伤。我们认为这种设计策略为开发新的人工抗氧化酶和生物催化材料提供了一条有趣的途径,在治疗多种慢性炎症性疾病方面具有潜在的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
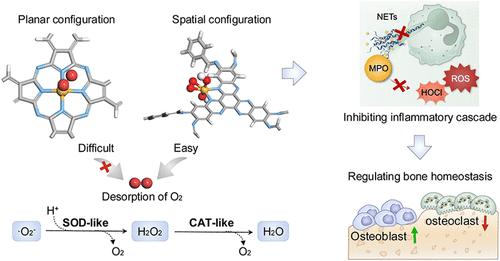
Spatial Configuration-Guided Design of Covalent Organic Framework-Based Artificial Metalloantioxidases for Inhibiting Inflammatory Cascades and Regulating Bone Homeostasis
Intense oxidative stress in bone tissues can trigger the hyperactivation of neutrophils, thereby causing inflammatory cascades to deteriorate bone homeostasis. Here, inspired by the catalytic centers of natural antioxidases, we introduce the spatial configuration-guided design of covalent organic framework (COF)-based artificial metalloantioxidases for inhibiting inflammatory cascades and regulating bone homeostasis. Specifically, the hexaiminohexaazatrinaphthalene COF with ruthenium coordination (S-HACOF-Ru), featuring electron-rich centers with a spatial configuration, demonstrates exceptional antioxidase-like reactive oxygen species (ROS) scavenging capabilities for efficiently mitigating the oxidative stress. As a result, S-HACOF-Ru efficiently prevents the generation of neutrophil extracellular traps and inhibits the release of myeloperoxidase (MPO). By preventing MPO-induced activation of nuclear factor-kappa B and inhibiting proinflammatory macrophage polarization, S-HACOF-Ru successfully blocks the neutrophil-macrophage inflammatory cascades. This intervention promotes bone homeostasis by a shift from bone resorption to tissue regeneration, which can efficiently inhibit alveolar bone loss in periodontal tissues and reverse cartilage damage in ankle joint cavities. We propose that this design strategy provides an intriguing avenue for developing new artificial antioxidases and biocatalytic materials with potential applications in treating a wide range of chronic inflammatory diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


