偶极-透射1,3-偶极环加成:模块化、多样性导向的多环生物碱支架合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本报告建立了偶极子传输1,3-偶极环加成方法,通过多样性导向的合成策略,可以快速、模块化和非对映选择性地构建特殊的生物碱支架。除了从简单的构建块中提供各种功能化吡咯利齐胺、吲哚利齐胺、喹诺利齐胺和吡唑啉框架外,这些工具还促进了生物碱天然产物异oretroncanol、elaeokanine A和grandiine D的正式合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
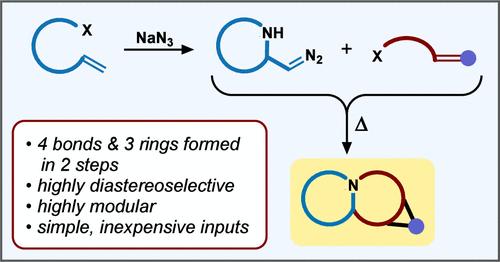
Dipole-Transmissive 1,3-Dipolar Cycloadditions: Modular, Diversity-Oriented Synthesis of Polycyclic Alkaloid Scaffolds
This report establishes dipole-transmissive 1,3-dipolar cycloaddition methodology that enables the rapid, modular, and diastereoselective construction of privileged alkaloid scaffolds via a diversity-oriented synthetic strategy. In addition to furnishing assorted functionalized pyrrolizidine, indolizidine, quinolizidine, and pyrazoline frameworks from simple building blocks, these tools facilitated formal syntheses of alkaloid natural products isoretronecanol, elaeokanine A, and grandisine D.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


