一个简单的方法来合成NiO纳米颗粒,既改善OER性能和超顺磁性行为
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
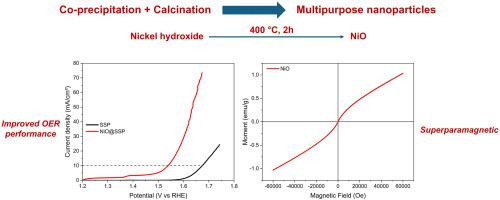
合成与昂贵的IrO2和RuO2性能相当或更好的析氧反应(OER)催化剂对于碱性电解的大规模应用至关重要。在这方面,NiO是此类应用的候选者之一,并且具有独特的磁性。在这项研究中,采用简单的共沉淀+煅烧方法合成了NiO纳米颗粒,获得了既适合改善OER活性又适合超顺磁性行为的颗粒。与草酸相比,NaOH制备的样品为单相,晶粒尺寸较小,导致Ni二次相的形成较少。在10 mA cm−2电流密度下,镀镍钢电极的OER过电位为309.5 mV, Tafel斜率为131.2 mV dec−1。这些结果与NiO的最佳OER性能相当。然而,少量二次镍相的存在对草酸制备的样品的OER性能有不利影响。此外,NiO电催化剂在310 mV过电位下的24 h OER操作中表现出优异的稳定性,电流密度变化极小。此外,使用NaOH制备的纳米颗粒具有较小的尺寸和超顺磁性,而使用草酸制备的纳米颗粒具有较大的晶粒尺寸,并与Ni次生相结合,从而具有铁磁性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A simple approach for the synthesis of NiO nanoparticles with both improved OER performance and superparamagnetic behaviour
Synthesis of oxygen evolution reaction (OER) catalysts with comparable or better properties than expensive IrO2 and RuO2 is crucial for large scale applications of alkaline water electrolysis. In this regard, NiO is one of the candidates for such applications and provides unique magnetic properties as well. In this study, NiO nanoparticles were synthesised using a simple co-precipitation + calcination approach to obtain particles with sizes suitable for both improved OER activity and superparamagnetic behaviour. The sample prepared with NaOH was single phase and had smaller crystallite size in comparison to the use of oxalic acid, which led to the formation of minor amount of Ni secondary phase. Electrodes prepared using nickel plated steel substrates with NiO catalyst exhibited an OER overpotential of 309.5 mV at 10 mA cm−2 current density, and the Tafel slope was calculated to be 131.2 mV dec−1. These results were comparable to the best OER performance for NiO. However, the presence of minor secondary nickel phase had a detrimental effect on the OER performance for the sample prepared with oxalic acid. Moreover, NiO electrocatalyst exhibited exceptional stability for 24 h of OER operation at 310 mV overpotential with minimal change in the current density. In addition, nanoparticles prepared using NaOH had smaller sizes and exhibited superparamagnetic behaviour while the use of oxalic acid led to a larger crystallite size and combined with the Ni secondary phase this led to a ferromagnetic behaviour.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


