人内源性逆转录病毒(herv)与胶质母细胞瘤的风险和预后相关。
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
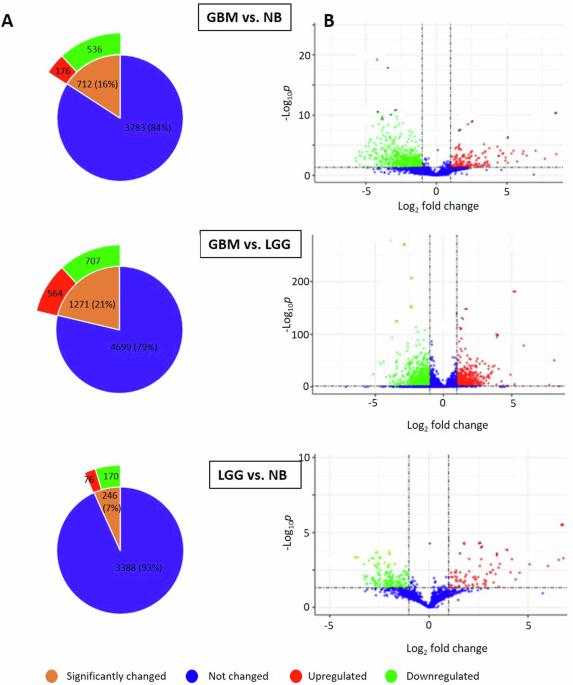
新出现的证据表明,人类内源性逆转录病毒(HERV)基因座的表达可能有助于多形性胶质母细胞瘤(GBM)疾病进展,或者是其生物标志物。然而,HERV表达与GBM恶性表型之间的关系尚不清楚。基于癌症基因组图谱(TCGA)的数据,我们进行了几项计算机分析,得出了胶质瘤的基因座特异性HERV转录组,发现在GBM与正常脑(NB)、GBM与低级别胶质瘤(LGG)、LGG与NB的比较中,有211个HERV显著失调。我们的分析支持了一种独特的HERV评分算法的开发,该算法可以区分GBM、LGG和NB。有趣的是,较低的HERV评分与较低的GBM生存率相关。然而,HERV评分在预测LGG生存或LGG进展为GBM方面不太可靠。功能预测分析将211个HERV基因座与18个电压门控钾通道基因联系起来。失调的herv和特定钾通道基因之间的功能联系可能有助于更好地了解GBM的发病机制、疾病进展和可能的耐药性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Human endogenous retroviruses (HERVs) associated with glioblastoma risk and prognosis
Emerging evidence suggests expression from human endogenous retrovirus (HERV) loci likely contributes to, or is a biomarker of, glioblastoma multiforme (GBM) disease progression. However, the relationship between HERV expression and GBM malignant phenotype is unclear. Applying several in silico analyses based on data from The Cancer Genome Atlas (TCGA), we derived a locus-specific HERV transcriptome for glioma that revealed 211 HERVs significantly dysregulated in the comparisons of GBM vs. normal brain (NB), GBM vs. low-grade glioma (LGG), and LGG vs. NB. Our analysis supported development of a unique HERV scoring algorithm that segregated GBM, LGG, and NB. Interestingly, lower HERV scores showed correlation with lower survival in GBM. However, HERV scores were less robust in predicting LGG survival or LGG progression to GBM. Functional prediction analysis linked the 211 HERV loci with 18 voltage-gated potassium channel genes. The functional link between dysregulated HERVs and specific potassium channel genes may contribute to better understanding of GBM pathogenesis, disease progression, and possibly drug resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


