靶向Lp-PLA2通过恢复心磷脂介导的线粒体自噬抑制矽肺中纤维化单核细胞来源的巨噬细胞。
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
单核细胞源性巨噬细胞(MoMacs)是引起肺纤维化的最重要的效应细胞。然而,MoMac在矽肺中的分化特征以及MoMac影响肺纤维化进展的机制尚不清楚。单细胞整合和空间转录组学分析显示,矽肺生态位由一个被鉴定为Spp1hiMacs的MoMacs亚群占据,该亚群在矽肺期间仍处于未成熟的过渡分化状态。本研究探讨了脂蛋白相关磷脂酶A2 (Lp-PLA2,由Pla2g7 -酰基辅酶a编码):溶心磷脂酰基转移酶-1 (ALCAT1)-心磷脂(CL)信号通路干扰Spp1hiMac分化诱导线粒体损伤的机制基础。我们证明,在sio2诱导的MoMacs中,Lp-PLA2通过激活ALCAT1诱导异常CL酰化,导致PINK1和LC3B的线粒体定位受损和线粒体自噬缺陷。同时,溶酶体功能障碍导致溶酶体蛋白组织蛋白酶B释放到细胞质中,涉及巨噬细胞M1和M2极化以及促炎和促纤维化途径的激活。此外,我们评估了Lp-PLA2抑制剂darapladib在小鼠模型中改善二氧化硅诱导的肺纤维化的功效。我们的发现增强了我们对矽肺发病机制的理解,并为开发靶向治疗提供了有希望的机会,以减缓纤维化进展并维持受影响个体的肺功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
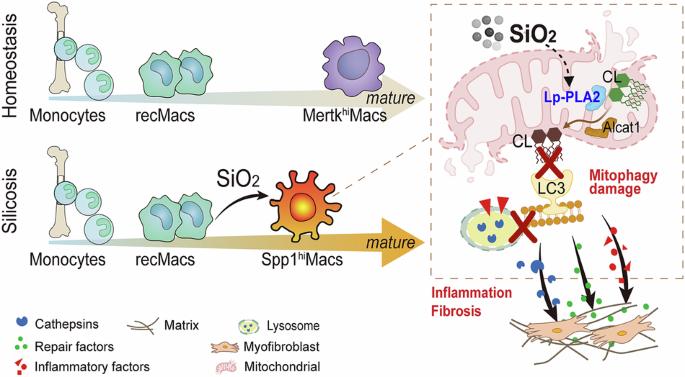
Targeting Lp-PLA2 inhibits profibrotic monocyte-derived macrophages in silicosis through restoring cardiolipin-mediated mitophagy
Monocyte-derived macrophages (MoMacs) are the most important effector cells that cause pulmonary fibrosis. However, the characteristics of MoMac differentiation in silicosis and the mechanisms by which MoMacs affect the progression of pulmonary fibrosis remain unclear. Integration of single-cell and spatial transcriptomic analyses revealed that the silicosis niche was occupied by a subset of MoMacs, identified as Spp1hiMacs, which remain in an immature transitional state of differentiation during silicosis. This study investigated the mechanistic foundations of mitochondrial damage induced by the lipoprotein-associated phospholipase A2 (Lp-PLA2, encoded by Pla2g7)–acyl-CoA:lysocardiolipin acyltransferase-1 (ALCAT1)–cardiolipin (CL) signaling pathway, which interferes with Spp1hiMac differentiation. We demonstrated that in SiO2-induced MoMacs, Lp-PLA2 induces abnormal CL acylation through the activation of ALCAT1, resulting in impaired mitochondrial localization of PINK1 and LC3B and mitochondrial autophagy defects. Simultaneously, lysosomal dysfunction causes the release of the lysosomal protein cathepsin B into the cytoplasm, which involves M1 and M2 macrophage polarization and the activation of proinflammatory and profibrotic pathways. Furthermore, we assessed the efficacy of the Lp-PLA2 inhibitor darapladib in ameliorating silica-induced pulmonary fibrosis in a murine model. Our findings enhance our understanding of silicosis pathogenesis and offer promising opportunities for developing targeted therapies to mitigate fibrotic progression and maintain lung function in affected individuals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


