LaCr1-xNixO3电催化氧还原过程中应变Ni(III)中心产生H2O2的异常增加
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
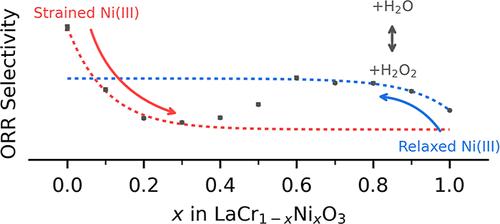
电化学氧还原反应(ORR)在能量储存和转化技术中起着至关重要的作用,并可能为工业上可持续产生H2O2提供一种可行的方法,但我们对催化剂结构改变反应选择性的方式的理解仍然不完整。利用x射线衍射和x射线吸收光谱对LaCr1-xNixO3进行分析,揭示了其结构演化的三个阶段。第一阶段的特征是Ni(II)和Ni(III)的混合存在于不同的LaCrO3晶格中,随着Ni含量的增加,Ni(III)的比例增加。Ni含量的持续增加诱导了大量的结构重排,最终导致从Pnma晶格到R3′c晶格的相变,所有这些都发生在平均Ni氧化态的变化可以忽略不计的情况下。相变后,随着晶格的压缩,Ni(III)含量继续增加。富Ni和富cr样品的不同反应选择性趋势建立了最大H2O2选择性的狭窄组成范围,在ORR过程中,LaCrO3晶格内存在高度应变的Ni(III)位点对H2O2的产生具有特别的选择性。我们将富cr样品的选择性增强归因于应变诱导的Ni(III)中心晶体场分裂的改变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Anomalous Increases in H2O2 Production by Strained Ni(III) Centers during Electrocatalytic Oxygen Reduction on LaCr1–xNixO3
The electrochemical oxygen reduction reaction (ORR) plays a critical role in energy storage and conversion technologies and may provide a viable method for sustainable generation of H2O2 for industrial use, but our understanding of the ways in which the catalyst structure alters reaction selectivity remains incomplete. Analysis of LaCr1–xNixO3 by X-ray diffraction and X-ray absorption spectroscopy reveals three stages of structural evolution across the composition series. The first stage is characterized by a blend of Ni(II) and Ni(III) residing within a distinct LaCrO3 lattice, with an increasing proportion of Ni(III) as Ni content increases. Continued increases in Ni content induce substantial structural rearrangement that culminates in a phase transition from a Pnma lattice to R3̅c, all of which occur with negligible changes in the average Ni-oxidation state. Following the phase transition, the Ni(III) content continues to increase as the lattice compresses. Diverging reaction selectivity trends for Ni-rich and Cr-rich samples establish a narrow composition range of maximal H2O2 selectivity, with highly strained Ni(III) sites present within a LaCrO3 lattice found to be particularly selective for H2O2 production during the ORR. We attribute the selectivity enhancements in Cr-rich samples to strain-induced alteration of the crystal field splitting for Ni(III) centers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


