(+)-亮氨酸和(−)-7-外亮氨酸的全合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这里,我们报道了从市售的piperidin-2-one开始,用8步实现(+)-亮色氨酸和(−)-7-亮色氨酸的简明全合成。我们的策略强调钯催化脱羧不对称烯丙基烷基化,以构建具有C20全碳季立体中心的δ-内酰胺。此外,顺式八氢呋喃[2,3-b]吡啶单元通过一锅协议包括还原和oxa- mannich型环化过程有效地构建。本文章由计算机程序翻译,如有差异,请以英文原文为准。
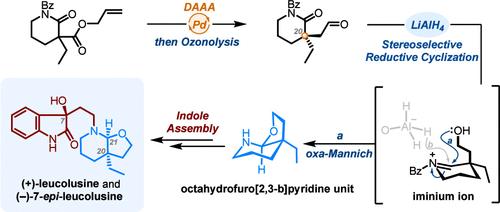
Total Synthesis of (+)-Leucolusine and (−)-7-epi-Leucolusine
Herein, we report the concise total syntheses of (+)-leucolusine and (−)-7-epi-leucolusine achieved in 8 steps starting from commercially available piperidin-2-one. Our strategy highlights a palladium-catalyzed decarboxylative asymmetric allylic alkylation for constructing the δ-lactam bearing a C20 all-carbon quaternary stereocenter. Additionally, the cis-fused octahydrofuro[2,3-b]pyridine unit was efficiently constructed via a one-pot protocol encompassing reduction and oxa-Mannich-type cyclization processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


