氯胺酮诱导的大鼠静态和动态功能连接变化受阿片受体和生物性别的调节。
IF 6.6
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
亚麻醉氯胺酮目前被用作各种神经精神疾病的速效治疗。然而,其治疗作用的机制基础仍不清楚,新出现的临床和临床前证据强调了阿片系统的潜在参与。我们使用了氯胺酮给药期间和之后获得的药理学功能超声成像数据,这些数据来自于纳曲酮(一种阿片受体拮抗剂)或载体预处理的雄性和雌性大鼠。我们发现氯胺酮诱导的功能连接变化是由阿片受体阻断调节的,并且这些反应依赖于生物性别。具体来说,纳曲酮性别依赖性地改变了内侧前额叶皮层(mPFC)内的连接模式,mPFC是大脑默认模式网络的关键节点,也是mPFC和其他功能节点之间的连接模式。此外,氯胺酮仅在雄性大鼠中产生阿片类药物依赖性转变,转向连接障碍和脑熵增加的状态。我们的发现为进一步研究氯胺酮作用的神经生理学基础以及与阿片受体的潜在性别特异性相互作用提供了依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
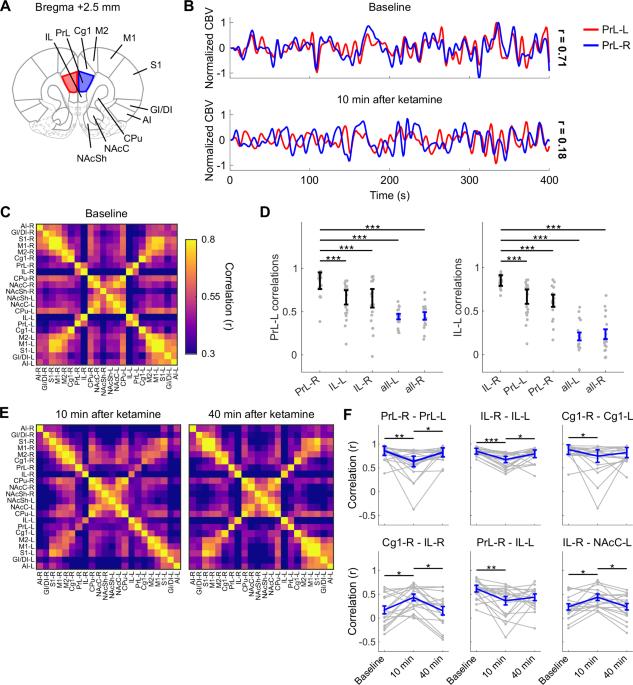
Ketamine-induced static and dynamic functional connectivity changes are modulated by opioid receptors and biological sex in rats
Subanesthetic ketamine is currently used as a rapid-acting treatment for varied neuropsychiatric disorders. However, the mechanistic underpinnings of its therapeutic action remain unclear, and emerging clinical and preclinical evidence highlights a potential involvement of the opioid system. We used pharmacological functional ultrasound imaging data acquired during and after ketamine administration in male and female rats pretreated with naltrexone, an opioid receptor antagonist, or vehicle. We found that ketamine-induced functional connectivity changes are modulated by opioid receptor blockade, and that these responses are dependent on biological sex. Specifically, naltrexone sex-dependently altered the connectivity patterns within the medial prefrontal cortex (mPFC), a key node of the brain’s default-mode network, and between the mPFC and other functional nodes. Furthermore, ketamine produced an opioid-dependent shift toward states of increased dysconnectivity and brain entropy in male rats only. Our findings warrant further investigation into the neurophysiological underpinnings of ketamine action and potential sex-specific interactions with opioid receptors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropsychopharmacology
医学-精神病学
CiteScore
15.00
自引率
2.60%
发文量
240
审稿时长
2 months
期刊介绍:
Neuropsychopharmacology is a reputable international scientific journal that serves as the official publication of the American College of Neuropsychopharmacology (ACNP). The journal's primary focus is on research that enhances our knowledge of the brain and behavior, with a particular emphasis on the molecular, cellular, physiological, and psychological aspects of substances that affect the central nervous system (CNS). It also aims to identify new molecular targets for the development of future drugs.
The journal prioritizes original research reports, but it also welcomes mini-reviews and perspectives, which are often solicited by the editorial office. These types of articles provide valuable insights and syntheses of current research trends and future directions in the field of neuroscience and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


