可持续保健和医学实验室:框架和举措之间全球合作的影响。
IF 2.1
3区 医学
Q2 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
摘要
气候变化是一项紧迫的全球挑战,需要采取紧急行动。《巴黎协定》和《联合国2030年议程》为减排和可持续性设定了明确的全球目标,将升温控制在1.5 °C以内。标准化组织之间的伙伴关系对于加快气候行动至关重要。日内瓦可持续发展中心(GSC)站在医疗保健可持续发展的最前沿,于2023年推出了可持续发展加速工具(SAT),以帮助医院评估其环境影响。国际联合委员会(JCI)与GSC合作,将可持续性纳入其认证标准,并将于2025年推出以SAT为基础的JCI-GSC医疗保健可持续性认证。为了加速实现可持续发展目标(SDG), ISO和联合国于2023年建立了战略合作伙伴关系,以创建首个全球可持续发展目标标准。ISO已经发布了IWA 42:22 22净零指南,并正在制定其首个全球净零标准,将于2025年发布。此外,ISO引入了气候变化修正案,将气候因素纳入包括ISO 9001在内的30多个现有管理体系标准。由于这些修订现在影响了所有新开发或修订的标准,ISO 15189的未来更新可能会纳入可持续性要求,特别是ISO 9001是ISO 15189的重要组成部分。仅减少医疗保健和医学实验室的运营排放并不能实现净零排放。在解决能源、药品和医疗设备的供应链排放问题时,必须采取全行业的方法。应对气候变化需要跨部门的协调努力,因此国际合作不可或缺。本综述重点介绍了通过协作而形成的关键全球框架、标准、指南和倡议,以帮助医疗保健组织(包括医学实验室)提高可持续性和气候适应能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
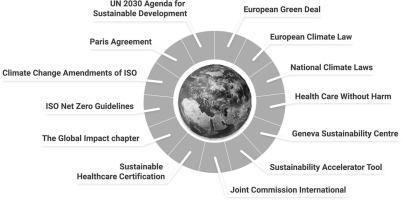
Sustainable healthcare and medical laboratories: The impact of global collaborations between frameworks and initiatives
Climate change is a pressing global challenge that requires urgent action. The Paris Agreement and the 2030 Agenda of United Nations (UN) set clear global targets for emission reduction and sustainability to limit warming to 1.5 °C. Partnerships between standardization organizations are crucial in accelerating climate action. The Geneva Sustainability Centre (GSC) is at the forefront of healthcare sustainability, launching the Sustainability Accelerator Tool (SAT) in 2023 to help hospitals assess their environmental impact. In partnership with GSC, Joint Commission International (JCI) has integrated sustainability into its accreditation standards and will introduce the JCI-GSC Healthcare Sustainability Certification in 2025, built on the SAT. To accelerate the achievement of the Sustainable Development Goals (SDGs), ISO and the UN formed a strategic partnership in 2023 to create the first global SDG standard. ISO has published the IWA 42:2022 Net Zero Guidelines and is developing its first global Standard on Net Zero, set for release in 2025. Additionally, ISO introduced the Climate Change Amendments, embedding climate considerations into over 30 existing management system standards, including ISO 9001. With these amendments now shaping all newly developed or revised standards, future updates to ISO 15189 will likely incorporate sustainability requirements, especially as ISO 9001 is an essential part of ISO 15189. Reducing operational emissions from healthcare and medical laboratories alone will not achieve net zero. A sector-wide approach is essential, tackling supply chain emissions from energy, pharmaceuticals, and medical devices. Combating climate change requires a coordinated, cross-sector effort, making international collaboration indispensable. This review highlights key global frameworks, standards, guidelines, and initiatives that have evolved through collaboration to help healthcare organizations, including medical laboratories, to advance sustainability and climate resilience.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Clinical biochemistry
医学-医学实验技术
CiteScore
5.10
自引率
0.00%
发文量
151
审稿时长
25 days
期刊介绍:
Clinical Biochemistry publishes articles relating to clinical chemistry, molecular biology and genetics, therapeutic drug monitoring and toxicology, laboratory immunology and laboratory medicine in general, with the focus on analytical and clinical investigation of laboratory tests in humans used for diagnosis, prognosis, treatment and therapy, and monitoring of disease.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


