同手性与外消旋2D共价有机框架
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
利用手性π共轭结构单元合成同手性二维共价有机框架(2D COFs)具有挑战性,因为手性单元经常导致堆叠相互作用错位。在这项工作中,我们将螺旋手性引入到二维COFs中,使用构型稳定的对映纯和外消旋[5]螺旋烯作为连接剂,在二维[5]螺旋ofs的骨架中作为粉末和薄膜。通过与1,3,5-三甲酰苯(TFB)或1,3,5-三甲酰间苯三酚(TFP)的缩合,我们的方法能够有效地形成一组同手性和外消旋的2D [5]HeliCOFs。所得的碳基晶体和多孔骨架具有明显的结构特征,并且在同手性和外消旋对应物之间具有不同的性质。螺旋手性传播到晶体框架的主干,导致在远红色可见光谱中观察到先进的手性,以及与外消旋框架相比不那么紧凑的结构。高序π共轭晶格的生长控制了[5]HeliCOFs薄膜的光致发光特性。本研究提供了对一般手性框架形成的见解,并将Liebisch-Wallach规则扩展到二维COFs。本文章由计算机程序翻译,如有差异,请以英文原文为准。
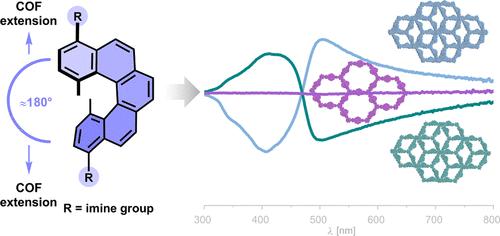
Homochiral versus Racemic 2D Covalent Organic Frameworks
The synthesis of homochiral two-dimensional covalent organic frameworks (2D COFs) from chiral π-conjugated building blocks is challenging, as chiral units often lead to misaligned stacking interactions. In this work, we introduce helical chirality into 2D COFs using configurationally stable enantiopure and racemic [5]helicenes as linkers in the backbone of 2D [5]HeliCOFs as powders and films. Through condensation with 1,3,5-triformylbenzene (TFB) or 1,3,5-triformylphloroglucinol (TFP), our approach enables the efficient formation of a set of homochiral and racemic 2D [5]HeliCOFs. The resulting carbon-based crystalline and porous frameworks exhibit distinct structural features and different properties between homochiral and racemic counterparts. Propagation of helical chirality into the backbone of the crystalline frameworks leads to the observation of advanced chiroptical properties in the far-red visible spectrum, along with a less compact structure compared with the racemic frameworks. Homogeneous thin films of [5]HeliCOFs disclosed photoluminescent properties arising from the controlled growth of highly ordered π-conjugated lattices. The present study offers insight into general chiral framework formation and extends the Liebisch–Wallach rule to 2D COFs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


