在混合手性多肽中控制波纹β片与褶皱β片的设计指南
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
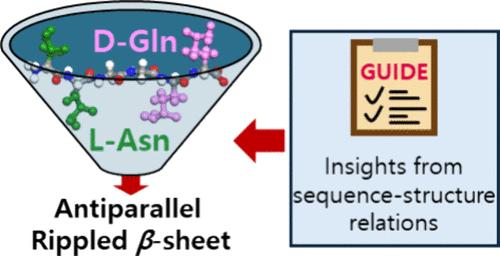
解码氨基酸序列如何决定结构有助于设计功能蛋白、高级生物材料和选择性低副作用药物。波浪形β薄片理论是鲍林和科里在1953年提出的,直到最近才开始获得实验支持。然而,对波纹β薄片的研究仍然有限,我们对它们何时以及如何发生的理解仍然存在空白。为了了解序列与波纹β片形成倾向之间的关系,我们进行了分子动力学(MD)和密度泛函理论(DFT)模拟,以预测六种形成平行或反平行的波纹或褶皱β片的系统的能量学。值得注意的是,在每个系统的这四种可能结构中,预测的具有最低能量的结构与实验观察到的单一情况一致!为了理解为什么这种形式受到青睐,我们研究了所有六种体系的局部结构,特别关注氢键(h键)在稳定中的作用。在每个系统中,肽始终采用一种基序,使其能够在主干之间形成最大数量的氢键,即使在修饰时也是如此,并且由具有混合手性的单一组分或环状肽组成。我们发现非手性甘氨酸-甘氨酸桥作为缬氨酸残基之间的间隔剂,有效地降低了侧链之间的位阻。此外,我们得出结论,环状肽的结构在无水环境下由分子内氢键稳定。我们的发现为序列如何影响β-薄片构象提供了更深入的见解,使我们能够为新肽的首选结构提出指导方针。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design Guidelines to Control Rippled β-Sheets versus Pleated β-Sheets in Mixed-Chirality Peptides
Decoding how amino acid sequences determine structure facilitates the design of functional proteins, advanced biomaterials, and selective, low-side-effect drugs. The rippled β-sheet, theorized by Pauling and Corey in 1953, has only recently begun to gain experimental support. However, research on rippled β-sheets remains limited, leaving gaps in our understanding of when and how they occur. To understand the relationship between sequences and rippled β-sheet formation propensities, we carried out molecular dynamics (MD) and density functional theory (DFT) simulations to predict the energetics for six systems of forming either a rippled or pleated β-sheets that are ordered either parallel or antiparallel. Notably, among these four possible structures of each system, the structure predicted to have the lowest energy agrees with the single case observed experimentally! To understand why this form is favored, we investigate the local structures of all six systems, with particular attention to the role of hydrogen bonds (H-bonds) in stabilization. In each system, the peptide consistently adopts a motif that allows it to form the maximum number of H-bonds between backbones, even when amidated, and composed of a single-component with mixed chirality or a cyclic peptide. We find that an achiral glycine–glycine bridge acts as a spacer between valine residues, effectively reducing steric hindrance between side chains. Furthermore, we conclude that the structures of cyclic peptides are stabilized by intramolecular H-bonds in an anhydrous environment. Our findings provide deeper insights into how sequences influence β-sheet conformations, enabling us to propose guidelines for the preferred structures of novel peptides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


