NFE2L3调节结肠中炎症和氧化应激相关基因。
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-05-11
DOI:10.1016/j.bbamcr.2025.119987
引用次数: 0
摘要
导致炎症性肠病(IBD)的分子机制仅被部分理解。我们通过右旋糖酐硫酸钠(DSS)诱导炎症,研究了转录因子NFE2L3在小鼠结肠炎模型中的作用。我们通过组织学分析和粪便中炎症标志物脂钙素-2 (LCN2)水平升高证实了炎症的存在。我们发现,与野生型小鼠相比,Nfe2l3-/-小鼠的Lcn2转录水平明显升高。我们进一步发现,在DSS治疗的野生型小鼠中,Nfe2l3 mRNA的表达减少。我们将NFE2L3结合伙伴MAFF和MAFK的ENCODE ChIP数据与已知的IBD和DSS效应物交叉对照,确定Stat1、Hmox1和Slc7a11是潜在的NFE2L3靶点。这些蛋白在结肠炎期间分别被诱导抑制免疫反应、减少氧化应激和触发铁下垂。我们分析了候选靶点,并观察到野生型DSS处理后它们的蛋白表达增加,但在Nfe2l3-/-小鼠中没有。此外,在缺乏DSS的情况下,我们观察到Nfe2l3-/-小鼠中pSTAT1和SLC7A11蛋白的基础水平增加。这些数据表明,在炎症性肠病(IBD)期间,NFE2L3转录因子将微环境启动到促炎症准备状态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
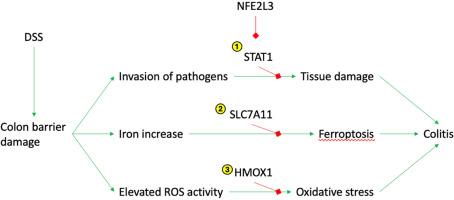
NFE2L3 regulates inflammation and oxidative stress-related genes in the colon
The molecular mechanisms leading to inflammatory bowel disease (IBD) are only partially understood. We investigated the role of the transcription factor NFE2L3 in a mouse model of colitis by inducing inflammation using dextran sodium sulfate (DSS). We confirmed the presence of inflammation by histological analysis and elevated levels of the inflammation marker lipocalin-2 (LCN2) in the stool. We found that Lcn2 transcript levels are significantly less elevated in Nfe2l3−/− mice than wild type mice. We further showed a reduction of Nfe2l3 mRNA, in wildtype mice upon DSS treatment. We cross referenced ENCODE ChIP data of NFE2L3 binding partners MAFF and MAFK with known IBD and DSS effectors and identified Stat1, Hmox1, and Slc7a11 as potential NFE2L3 targets. These proteins are induced during colitis to suppress the immune response, reduce oxidative stress, and trigger ferroptosis, respectively. We analyzed the candidate targets and observed an increase in their protein expression upon DSS treatment in wild type but not in Nfe2l3−/− mice. Furthermore, in the absence of DSS, we observed an increase in the basal levels of pSTAT1 and SLC7A11 proteins in Nfe2l3−/− mice. These data suggest that the NFE2L3 transcription factor primes the microenvironment towards a pro-inflammatory ready state during inflammatory bowel disease (IBD).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


