接受溶瘤腺病毒TILT-123治疗的患者外周血单个核细胞的单细胞分析揭示了基线免疫状态作为治疗结果的预测因子。
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
溶瘤腺病毒Ad5/3-E2F-d24-hTNFa-IRES-hIL2 (TILT-123, igrelimogene litadenorevec)有望成为一种能够引起肿瘤消退和激活宿主免疫的治疗剂。一项I期临床研究TUNIMO (NCT04695327)评估了其作为单药治疗各种实体肿瘤患者的安全性。通过外周血的单细胞分析,我们确定了区分应答者和无应答者的不同免疫特征。具体来说,在基线时,应答者表现出增强的细胞毒性标记和更强的免疫细胞通信网络。此外,较高的基线CD16+单核细胞与改善的生存率相关,而升高的调节性T细胞预示着不良反应。T细胞和B细胞评估显示了截然不同的模式:应答者显示出更多的T细胞,并预测对腺病毒和肿瘤抗原的特异性,而升高的总记忆B细胞,无论特异性如何,都预示着较差的生存率。一些T和B细胞受体片段与先前报道的其他病毒感染相匹配,表明可能存在交叉反应性免疫反应。这些发现强调,全面的外周血生物标志物分析不仅应包括细胞频率,还应包括转录变化以及细胞和体液免疫的独特模式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
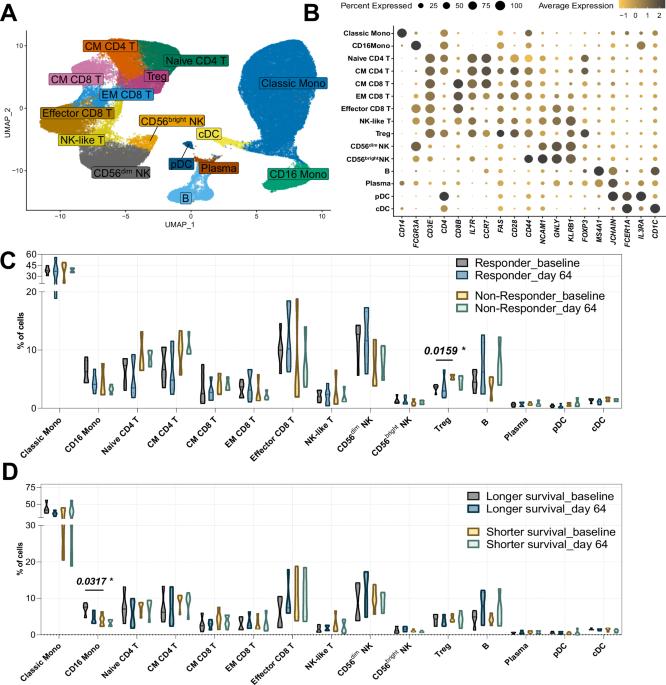
Single-cell profiling of peripheral blood mononuclear cells from patients treated with oncolytic adenovirus TILT-123 reveals baseline immune status as a predictor of therapy outcomes
Oncolytic adenovirus Ad5/3-E2F-d24-hTNFa-IRES-hIL2 (TILT-123, igrelimogene litadenorepvec) shows promise as a therapeutic agent capable of causing tumor regression and activating host immunity. A phase I clinical study TUNIMO (NCT04695327) assessed its safety as monotherapy in patients with various solid tumors. Through single-cell profiling of peripheral blood, we identified distinct immunological features distinguishing responders from non-responders. Specifically, at baseline, responders demonstrated enhanced cytotoxic markers and stronger immune cell communication networks. Moreover, higher baseline CD16+ monocytes correlated with improved survival, while elevated regulatory T cells predicted poor response. T and B cell evaluation revealed contrasting patterns: responders showed higher numbers of T cells with predicted specificity to both adenovirus and tumor antigens, while elevated total memory B cells, regardless of specificity, predicted poor survival. Several T and B cell receptor segments matched those previously reported in other viral infections, suggesting possible cross-reactive immune responses. These findings emphasize that comprehensive biomarker analysis of peripheral blood should include not only cell frequencies but also transcriptional changes and distinct patterns of cellular and humoral immunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


