多受体酪氨酸激酶激活的酪氨酸激酶ACK1通过调控PI3K/AKT/mTOR和RAF/MAPK信号通路促进间皮瘤的增殖和侵袭。
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
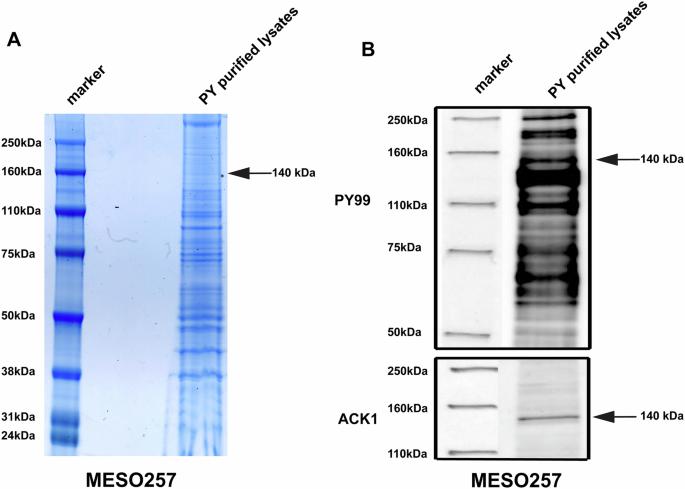
在间皮瘤亚群中已经发现了受体酪氨酸激酶(rtk)、EGFR、MET和AXL的激活,这表明酪氨酸激酶(TKs)可能是这种化疗耐药和高致死性癌症的治疗靶点。本研究采用磷酸酪氨酸免疫亲和纯化和串联质谱法在间皮瘤细胞中鉴定了活化的TKs,并对其生物学功能进行了评价。结果显示,非rtk激活的cdc42激酶1 (ACK1)在9个间皮瘤细胞系中的8个和18个间皮瘤活检中的15个中高表达和激活,而在正常间皮瘤细胞中不表达和激活。这种ACK1激活反过来由EGFR、MET和AXL的集体激活驱动。小分子抑制剂(AIM-100)或RNAi灭活ACK1在4个因G1阻滞和异种移植模型引起的间皮瘤培养中具有抗增殖、抗迁移和促凋亡作用。这些反应源于PI3K/AKT/mTOR和RAF/MAPK通路的抑制,细胞周期蛋白A和细胞周期蛋白D1的抑制,以及细胞周期检查点TP53、CDKN1A (p21)和CDKN1B (p27)的上调。AIM-100、顺铂(CIS)和培美曲塞(PEM)联合治疗对间皮瘤反应(细胞凋亡、增殖抑制、迁移和侵袭抑制)的影响比仅使用其中一种或两种药物更大。目前的研究结果确定ACK1是一个单一的下游靶点,可以抑制这些多受体酪氨酸激酶(EGFR, MET和AXL)在间皮瘤中的致癌程序,并强调ACK1抑制,可能与PEM和CIS联合,值得评估作为间皮瘤的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The activated tyrosine kinase ACK1 by multiple receptor tyrosine kinases promotes proliferation and invasion of mesothelioma via regulation of PI3K/AKT/mTOR and RAF/MAPK signaling pathways
Activation of the receptor tyrosine kinases (RTKs) EGFR, MET, and AXL has been described in subsets of mesothelioma, suggesting that tyrosine kinases (TKs) might represent therapeutic targets in this chemotherapy resistant and highly lethal cancer. In the present study, activated TKs were identified in mesothelioma cells by phosphotyrosine immunoaffinity purification and tandem mass spectrometry, and biological functions were evaluated. The results showed that non-RTK activated-CDC42 kinase 1 (ACK1) was highly expressed and activated in 8 of 9 mesothelioma cell lines and 15 of 18 mesothelioma biopsies, but not in normal mesothelial cells. This ACK1 activation was in turn driven by the collective activation of EGFR, MET, and AXL. ACK1 inactivation by either a small molecule inhibitor (AIM-100) or RNAi had anti-proliferative, anti-migration, and pro-apoptotic effects in four mesothelioma cultures due to G1 arrest and xenograft model. These responses resulted from inhibition of the PI3K/AKT/mTOR and RAF/MAPK pathways, inhibition of cyclin A and cyclin D1, and up-regulation of cell cycle checkpoints TP53, CDKN1A (p21), and CDKN1B (p27). Combination treatment with AIM-100, cisplatin (CIS), and pemetrexed (PEM) had greater impact on mesothelioma response (apoptosis, proliferation arrest, and inhibition of migration and invasion) compared to administering only one or two of these agents. The current findings identify ACK1 as a single downstream target that can be inhibited to stymie these multiple receptor tyrosine kinase (EGFR, MET, and AXL) oncogenic programs in mesothelioma, and highlight that ACK1 inhibition, potentially in combination with PEM and CIS, warrants evaluation as a therapeutic strategy in mesothelioma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


