GPX4降解通过伴侣介导的自噬参与热应激诱导的肝损伤
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-05-12
DOI:10.1016/j.bbamcr.2025.119988
引用次数: 0
摘要
热应激(HS)是一个重大的健康问题,对人类和动物的健康都有不利影响,特别是由于其中心代谢作用而影响肝功能。本研究探讨了hs诱导肝损伤的机制,重点关注铁下沉的作用,铁下沉是一种铁依赖性的细胞死亡形式,以脂质过氧化和细胞铁积累为特征。通过小鼠和细胞HS模型,结果表明HS通过铁下沉诱导肝损伤,其表现为丙二醛(MDA)、氧化谷胱甘肽(GSSG)和铁水平升高,谷胱甘肽(GSH)和谷胱甘肽过氧化物酶4 (GPX4)表达降低。ferroptosis抑制剂Ferrostatin-1 (fer1)可有效减轻hs诱导的肝损伤,降低氧化应激,恢复GPX4水平。此外,HS通过伴侣介导的自噬(CMA)途径促进GPX4的溶酶体降解,该途径受热休克同源蛋白70 (HSC70)和溶酶体相关膜蛋白2A (LAMP2A)的调控。肝细胞中敲低LAMP2A可显著抑制hs诱导的GPX4降解,证实了CMA在这一过程中的关键作用。使用HSC70抑制剂凋亡唑(apoptosis zole)或溶酶体抑制剂巴菲霉素A1 (Baf-A1)抑制CMA,进一步减轻hs诱导的铁上吊和肝损伤。这些发现强调了cma介导的GPX4降解在hs诱导的铁下垂和肝损伤中的关键作用,为减轻hs相关的肝损伤提供了潜在的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
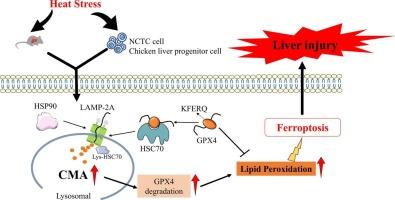
GPX4 degradation contributes to heat stress-induced liver injury via chaperone-mediated autophagy
Heat stress (HS) is a significant health concern that adversely affects both human and animal health, particularly impacting liver function due to its central metabolic role. This study investigated the mechanisms underlying HS-induced liver injury, focusing on the role of ferroptosis, an iron-dependent form of cell death characterized by lipid peroxidation and cellular iron accumulation. Using mouse and cellular HS models, the results demonstrated that HS induced liver injury through ferroptosis, as evidenced by increased levels of malondialdehyde (MDA), oxidized glutathione (GSSG), and iron, alongside decreased glutathione (GSH) and glutathione peroxidase 4 (GPX4) expression. The ferroptosis inhibitor Ferrostatin-1 (Fer-1) effectively mitigated HS-induced liver damage, reducing oxidative stress and restoring GPX4 levels. Furthermore, HS promoted the lysosomal degradation of GPX4 via the chaperone-mediated autophagy (CMA) pathway, which was regulated by heat shock cognate protein 70 (HSC70) and lysosome-associated membrane protein 2A (LAMP2A). Knockdown of LAMP2A in hepatocytes significantly suppressed HS-induced GPX4 degradation, confirming the critical role of CMA in this process. Inhibition of CMA using Apoptozole, an HSC70 inhibitor, or Bafilomycin A1 (Baf-A1), a lysosomal inhibitor, further attenuated HS-induced ferroptosis and liver injury. These findings highlight the critical role of CMA-mediated GPX4 degradation in HS-induced ferroptosis and liver injury, providing potential therapeutic targets for mitigating HS-related liver damage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


