e -钙粘蛋白机械转导通过诱导adam介导的配体脱落激活上皮单层中的EGFR-ERK信号
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
细胞的行为受来自其局部环境的信号控制,包括施加在细胞上的机械力。力是由机械敏感蛋白传导的,它可以影响信号级联反应,而信号级联反应也被生长因子激活。我们研究了机械和生化信号在上皮单层细胞内信号网络调控中的串扰。对机械应变作用下上皮单层的磷酸化蛋白质组学和转录组学分析显示,表皮生长因子受体(EGFR)下游的细胞外信号调节激酶(ERK)的激活是菌株诱导的主要信号事件。菌株诱导的EGFR-ERK信号依赖于机械敏感的e -钙粘蛋白粘附。接近标记表明,金属蛋白酶ADAM17(一种介导可溶性EGFR配体脱落的酶)与E-cadherin密切相关。我们开发的用于监测ADAM介导的脱落的探针表明,机械应变诱导ADAM激活。机械诱导的ADAM激活对于机械敏感的、依赖e -钙粘蛋白的EGFR-ERK信号传导至关重要。总之,我们的数据表明,由e -钙粘蛋白粘附转导的机械应变触发EGFR配体的脱落,从而刺激下游ERK活性。我们的研究结果说明了机械信号和生化配体如何在线性信号级联中运作。本文章由计算机程序翻译,如有差异,请以英文原文为准。
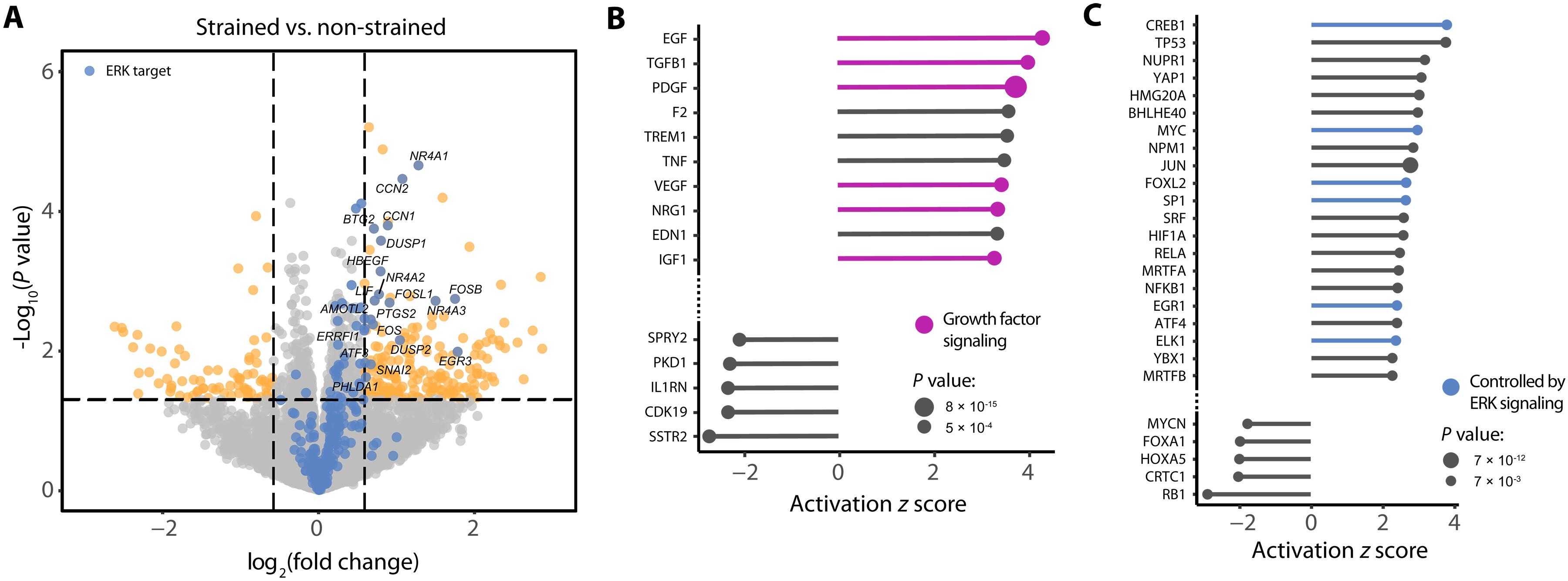
E-cadherin mechanotransduction activates EGFR-ERK signaling in epithelial monolayers by inducing ADAM-mediated ligand shedding
The behavior of cells is governed by signals originating from their local environment, including mechanical forces exerted on the cells. Forces are transduced by mechanosensitive proteins, which can impinge on signaling cascades that are also activated by growth factors. We investigated the cross-talk between mechanical and biochemical signals in the regulation of intracellular signaling networks in epithelial monolayers. Phosphoproteomic and transcriptomic analyses on epithelial monolayers subjected to mechanical strain revealed the activation of extracellular signal–regulated kinase (ERK) downstream of the epidermal growth factor receptor (EGFR) as a predominant strain-induced signaling event. Strain-induced EGFR-ERK signaling depended on mechanosensitive E-cadherin adhesions. Proximity labeling showed that the metalloproteinase ADAM17, an enzyme that mediates shedding of soluble EGFR ligands, was closely associated with E-cadherin. A probe that we developed to monitor ADAM-mediated shedding demonstrated that mechanical strain induced ADAM activation. Mechanically induced ADAM activation was essential for mechanosensitive, E-cadherin–dependent EGFR-ERK signaling. Together, our data demonstrate that mechanical strain transduced by E-cadherin adhesion triggers the shedding of EGFR ligands that stimulate downstream ERK activity. Our findings illustrate how mechanical signals and biochemical ligands can operate within a linear signaling cascade.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


