NFE2L2 (NRF2)转录因子控制着参与肌内膜细胞氧化应激反应和炎症的基因
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-05-11
DOI:10.1016/j.bbamcr.2025.119985
引用次数: 0
摘要
子宫肌层是子宫的平滑肌层,在分娩时调节子宫收缩。我们用促炎细胞因子白细胞介素-1 β (IL1B)处理PHM1-31子宫内膜细胞,并测量了活性氧(ROS)的显著增加。我们发现IL1B诱导NFE2L2 (NRF2)转录因子水平。我们进一步表明,siRNA介导的NFE2L2敲低导致ROS显著增加。NFE2L2的下调导致血红素加氧酶-1 (HMOX1)和醛酮还原酶家族1成员B (AKR1B)在转录物和蛋白水平上的降低,无论IL1B是否存在。NFE2L2敲低也导致铁蛋白重链1 (FTH1) mRNA表达降低,但仅在IL1B暴露时,而FTH1蛋白在基础和IL1B处理条件下均下调。我们证实NFE2L2直接结合到这些靶标的调控区域。先前的报道将HMOX1和FTH1与氧化应激反应联系起来,将AKR1B1与前列腺素合成联系起来。我们的数据表明,NFE2L2通过调节HMOX1、FTH1和AKR1B1在肌层细胞中的表达,作为炎症和氧化应激信号的关键调节剂。虽然HMOX1和FTH1已经在氧化应激反应中发挥了作用,但我们的研究结果发现AKR1B1是肌层细胞中NFE2L2的新靶点,这表明转录因子在前列腺素代谢中起作用。因此,NFE2L2将炎症和氧化应激反应与控制子宫肌细胞功能和分娩的关键途径联系起来,突出了它们作为治疗感染诱发早产的治疗靶点的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
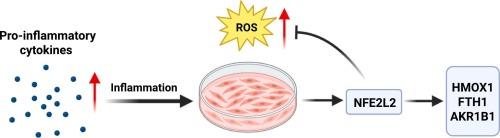
The NFE2L2 (NRF2) transcription factor controls genes involved in the oxidative stress response and inflammation in myometrial cells
The myometrium is the smooth muscle layer of the uterus, which mediates uterine contractions during labor. We treated PHM1–31 myometrial cells with the proinflammatory cytokine interleukin-1 beta (IL1B) and measured a significant increase in reactive oxygen species (ROS). We found that IL1B induces NFE2L2 (NRF2) transcription factor levels. We further showed that siRNA mediated knockdown of NFE2L2 results in a significant increase in ROS. Downregulation of NFE2L2 leads to a decrease of heme oxygenase-1 (HMOX1) and aldo-keto reductase family 1 member B (AKR1B) at the transcript and protein level both in the absence and presence of IL1B. NFE2L2 knockdown also results in reduced ferritin heavy chain 1 (FTH1) mRNA expression, but only upon IL1B exposure, while FTH1 protein is downregulated both under basal and IL1B treatment conditions. We confirmed that NFE2L2 directly binds to the regulatory regions of these targets. Previous reports have linked HMOX1 and FTH1 to the oxidative stress response, and AKR1B1 to prostaglandin synthesis. Our data demonstrate that NFE2L2 functions as a key regulator of inflammatory and oxidative stress signaling through the regulation of HMOX1, FTH1, and AKR1B1 expression in myometrial cells. While HMOX1 and FTH1 have established roles in oxidative stress responses, our findings identify AKR1B1 as a novel target of NFE2L2 in myometrial cells, suggesting a role for the transcription factor in prostaglandin metabolism. Thus, NFE2L2 links inflammation and the oxidative stress response to critical pathways that control myometrial cell function and parturition, highlighting their potential as therapeutic targets for treating infection-induced preterm labor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


