分子镊子阻断蛋白质机器的功能孔
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们提出对称多价镊子作为第一类超分子元件设计覆盖和功能阻断蛋白质孔。作为模型,我们选择了酶p97,这是一种六聚体的aaa -ATP酶,通过将底物蛋白穿过对称p97六聚体中心的孔和通道,通过ATP水解来展开或分离底物蛋白。在合理的设计方法下,我们在分子模型的指导下开发了一类新的p97抑制剂。它们通过适当定位的分子镊子在孔的入口处与赖氨酸残基对接。配体结合的同时,内置的结合敏感发光团会产生荧光,作为亲和力测定的传感器。我们进一步证实了与p97的特异性相互作用,以及atp酶活性和蛋白质底物展开的伴随抑制,使用了一系列生物物理方法和最先进的生化分析。通过诱变也验证了特异性结合,表明p97功能的抑制是通过阻断孔入口介导的。特别是c3对称的多价镊子能有效抑制atp酶活性和蛋白质底物加工,这与对接位点的对称性一致。我们的数据独立地证实了底物穿线是p97蛋白展开的一种机制,并强调了多价镊子是一种靶向各种蛋白质孔隙的超分子策略。由于p97及其相关蛋白机器对蛋白质质量控制和细胞存活至关重要,因此新的孔结合物可能为对抗疾病和药物开发开辟新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
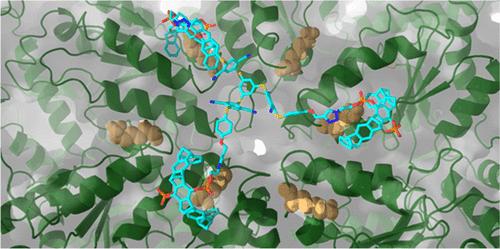
Molecular Tweezers Block the Functional Pore of a Protein Machine
We present symmetric multivalent tweezers as the first class of supramolecular elements designed to cover and functionally block a protein pore. As a model, we chose the enzyme p97, a hexameric AAA-ATPase that unfolds or segregates substrate proteins by threading them through a pore and channel at the center of the symmetric p97 hexamer fueled by ATP hydrolysis. In a rational design approach, we developed a new class of p97 inhibitors, guided by molecular modeling. These dock onto lysine residues at the entry of the pore via appropriately positioned molecular tweezers. Ligand binding was accompanied by induction of fluorescence of the built-in binding sensitive luminophores which served as a sensor for affinity determination. We further confirmed specific interaction with p97 as well as concomitant inhibition of ATPase activity and protein substrate unfolding using an array of biophysical methods and state-of-the art biochemical assays. Specific binding was also validated by mutagenesis, demonstrating that inhibition of p97 function was mediated by blocking the pore entrance. Especially C3-symmetric multivalent tweezers potently inhibited ATPase activity and protein substrate processing consistent with the symmetry of the docking site. Our data independently confirm substrate threading as a mechanism for protein unfolding by p97 and highlight multivalent tweezers as a supramolecular strategy to target pores in various proteins. Since p97 and related protein machines are vital for protein quality control and cell survival, the new pore binders may open a new approach to combat diseases and be employed in drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


