稀有气体修饰平面四配位氧
IF 2.9
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在平面四配位原子中,平面四配位氧原子(ptO)是罕见的。本研究建立了惰性气体支撑平面四配位氧原子(ptO)的第一个例子。高水平从头计算表明,He和Ne形成完美的平面D4h ptO结构作为全局最小值。详细的电子结构分析表明,中心氧原子通过强共价相互作用成键。目前的发现说明了稀有气体原子稳定平面四配位氧原子的能力,并将促进对涉及氧的非常规化学键的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
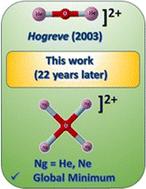
Noble gas decorated planar tetracoordinate oxygen†
Among planar tetracoordinate atoms, planar tetracoordinate oxygen atoms (ptO) are rare. The present study establishes the first example of noble gas supported planar tetracoordinate oxygen atoms (ptO). High level ab initio calculations indicate that He and Ne form perfect square planar D4h ptO structures as the global minimum. Detailed electronic structure analyses reveal that the central oxygen atom is bonded through strong covalent interactions. The present finding illustrates the ability of noble gas atoms in stabilizing planar tetracoordinate oxygen atoms and will advance the understanding of unconventional chemical bonding involving oxygen.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Physical Chemistry Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
5.50
自引率
9.10%
发文量
2675
审稿时长
2.0 months
期刊介绍:
Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


