C-C键在石杉碱生物合成后期通过酶逆转录- aza - prins反应发生
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在化学合成中对新型酶催化反应的需求刺激了许多新自然反应的发展。此外,对生物合成途径的详细分析可以揭示前所未有的化学/酶机制。在这项研究中,我们重新研究了2-氧戊二酸依赖的双加氧酶Pt2OGD-1在石杉碱生物碱合成中的催化机制。我们的实验和计算研究发现,在生物碱支架的哌啶环中,存在一种以前未知的酶催化的C-C键断裂,类似于氧化逆转录-aza- prins反应。在这里,这种转变是由抽氢开始的,然后在杂环的4位进行电子转移,触发开环,最后导致一个碳原子作为甲醛损失。这一发现扩大了反应的工具箱,增强了我们对这些酶的理解,并可能促进它们在生物技术生产相关生物碱支架以及具有类似活性的生物催化剂方面的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
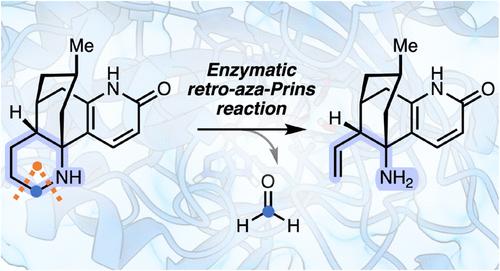
C–C Bond Cleavage in the Late-Stage Biosynthesis of Huperzine Alkaloids Occurs via Enzymatic Retro-Aza-Prins Reaction
The demand for novel enzyme-catalyzed reactions in chemical synthesis has spurred the development of many new-to-nature reactions. Additionally, detailed analysis of biosynthetic pathways can uncover unprecedented chemical/enzymatic mechanisms. In this study, we revisited the catalytic mechanism of the 2-oxoglutarate-dependent dioxygenase Pt2OGD-1, involved in the biosynthesis of huperzine alkaloids. Our experimental and computational investigations uncovered a previously unknown enzymatic C–C bond cleavage in the piperidine ring of the alkaloid scaffold, resembling an oxidative retro-aza-Prins reaction. Here, this transformation is initiated by hydrogen abstraction, followed by electron transfer at the 4-position of the heterocycle, triggering ring opening and finally resulting in the loss of a carbon atom as formaldehyde. This discovery expands the toolbox of reactions, enhances our understanding of these enzymes, and may facilitate their application in the biotechnological production of pharmaceutically relevant alkaloid scaffolds as well as the development of biocatalysts with similar activities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


