na -离子和Na-O2电池用稳定的natfsi型高浓度电解质
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
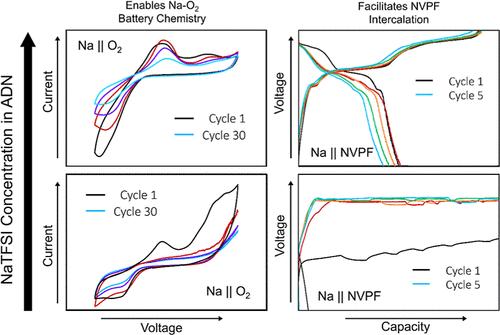
二(三氟甲磺酰基)亚胺钠(NaTFSI)和己二腈(ADN)作为钠离子电池的电解质具有高稳定性和安全性,但由于NaTFSI对Al的严重腐蚀以及Na金属对ADN的自发降解,在现代电池中无法使用。本文研究了NaTFSI-ADN电解质的电化学性质与浓度的关系。测量了NaTFSI-ADN的离子电导率和相图,并进行了分子动力学(MD)模拟,深入了解了电解质的溶液结构。循环伏安法(CV)实验发现,电解液的还原稳定性随浓度的增加而急剧增加,而线性扫描伏安法(LSV)和计时伏安法(CA)实验发现,TFSI对Al的寄生溶解随浓度的增加而降低。在钠离子电池中,4.4 M电解液增强还原性稳定性和抑制Al腐蚀的双重作用使得4.4 M电解液可以可逆地插入高压Na3V2(PO4)2F3 (NVPF)阴极多次循环,而标准1.0 M电解液没有观察到NVPF的插入。Na-O2电池也受益于高浓度的电解质,在Na-O2硬币电池的CV实验中显示出更长的寿命。浓缩NaTFSI-ADN电解质为钠电池提供了实际的好处,应该与进一步的稳定策略一起实施。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Stable NaTFSI-Based Highly Concentrated Electrolytes for Na-Ion and Na–O2 Batteries
Sodium bis(trifluoromethanesulfonyl)imide (NaTFSI) and adiponitrile (ADN) have attractive high stability and safety properties for application as electrolytes in Na-ion batteries, but are unusable in modern cells due to significant Al corrosion by NaTFSI, and spontaneous ADN degradation by Na metal. Herein, the electrochemical properties of NaTFSI–ADN electrolytes are investigated as a function of concentration. The ionic conductivity and phase diagram of NaTFSI–ADN is measured, and molecular dynamics (MD) simulations give insight into the solution structure of the electrolyte. The reductive stability of the electrolyte is found to increase drastically with concentration in cyclic voltammetry (CV) experiments, and the parasitic dissolution of Al by TFSI decreases with concentration in linear sweep voltammetry (LSV) and chronoamperometry (CA) tests. In Na-ion cells, the dual effect of reductive stability enhancement and Al corrosion suppression allows the 4.4 M electrolyte to reversibly intercalate high voltage Na3V2(PO4)2F3 (NVPF) cathodes for multiple cycles, while no NVPF intercalation is observed with the standard 1.0 M electrolyte. Na–O2 cells also benefit from the highly concentrated electrolyte, showing significantly longer lifetimes in CV experiments on Na–O2 coin cells. Concentrating NaTFSI–ADN electrolytes offers practical benefits to Na batteries and should be implemented with further stabilizing strategies going forward.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


