无催化剂聚聚氨酯共价自适应网络在后处理后显示完全交联密度恢复:由精心设计的仲胺合成的促进作用
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
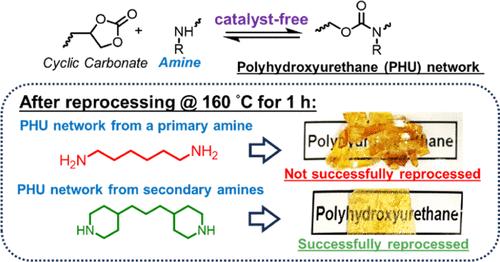
聚羟基聚氨酯(PHU)网络通常是用伯胺和环碳酸盐(cc)合成的,有或没有催化。相对于伯胺,由于空间位阻的增加,类似的仲胺对碳离子的反应性较低。因此,只有一篇利用仲胺和cc成功合成PHU网络的文献报道,没有对其动态特性进行评估。由伯多功能胺制成的PHU共价适应性网络(can)需要催化剂在适当的温度和时间框架下进行再处理,以最大限度地减少不必要的副反应。所有先前成功的PHU CAN合成和后处理报告都涉及催化剂。通过类比由仲胺制备的受阻脲键基can,我们假设具有相对低位阻的仲胺可以实现PHU can的无催化剂合成和高效的无催化剂后处理。小分子研究表明,n -己基甲胺(HMA)和哌啶(PiP)这两种具有有限位阻的单官能团仲胺与CCs反应时,其分数转化率非常高,但与具有较大位阻的仲胺反应时,分数转化率要低得多。小分子研究还表明,与基于伯胺的HU相比,基于hma和pip的羟基聚氨酯(HUs)在140°C下的无催化剂键交换率更高,这表明由精心设计的仲胺组成的PHU网络可能产生无催化剂的再加工性。我们用有限位阻的双官能仲胺,N,N ' -二甲基-1,6-己二胺(DMHDA)和4,4 ' -三亚甲基二哌啶(TmPiP)合成了PHU网络。这些PHU网络表现出更强的解离特性,在160°C时,它们的应力弛豫速度分别是基于伯胺的PHU网络的3倍和4倍。在160°C下压缩成型1.0 h,我们可以将无催化剂的dmhda和tmpip基PHU网络再加工成牢固的薄膜,但不能将无催化剂的叔胺基PHU网络再加工成薄膜。无催化剂的,基于仲胺的PHU can可再加工,交联密度和拉伸性能完全恢复。因此,本研究表明,由精心设计的多功能仲胺制成的无催化剂PHU can可以很容易地合成,并且具有强大的再处理能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Catalyst-Free Polyhydroxyurethane Covalent Adaptable Networks Exhibiting Full Cross-Link Density Recovery after Reprocessing: Facilitation by Synthesis with Well-Designed Secondary Amines
Polyhydroxyurethane (PHU) networks are generally synthesized using primary amines and cyclic carbonates (CCs), with or without catalysis. Relative to primary amines, analogous secondary amines are less reactive toward CCs due to increased steric hindrance. Consequently, there is only one literature report of a successful PHU network synthesis using secondary amines and CCs, with no assessment of dynamic character. PHU covalent adaptable networks (CANs) made from primary multifunctional amines require catalysts for reprocessing at moderate temperatures and time frames that minimize undesired side reactions. All prior reports of successful PHU CAN synthesis and reprocessing have involved catalysts. By analogy to hindered-urea-bond-based CANs made from secondary amines, we hypothesized that secondary amines with relatively low steric hindrance enable both catalyst-free synthesis and efficient catalyst-free reprocessing of PHU CANs. Small-molecule studies showed that two monofunctional secondary amines with limited steric hindrance, N-hexylmethylamine (HMA) and piperidine (PiP), reacted with CCs to very high fractional conversion, but reactions involving secondary amines with greater steric hindrance led to much lower fractional conversion. Small-molecule studies also revealed higher catalyst-free bond exchange rates at 140 °C in HMA-based and PiP-based hydroxyurethanes (HUs) compared to a primary-amine-based HU, suggesting that PHU networks made from well-designed secondary amines may yield catalyst-free reprocessability. We synthesized PHU networks from difunctional secondary amines with limited steric hindrance, N,N′-dimethyl-1,6-hexanediamine (DMHDA) and 4,4′-trimethylene dipiperidine (TmPiP). These PHU networks exhibited greater dissociative character and factors of 3- and 4-times faster stress relaxation at 160 °C, respectively, than a primary-amine-based PHU network. Using compression molding at 160 °C for 1.0 h, we could reprocess catalyst-free DMHDA-based and TmPiP-based PHU networks into well-consolidated films but not the catalyst-free primary-amine-based PHU network. The catalyst-free, secondary-amine-based PHU CANs were reprocessable with full recovery of the cross-link density and tensile properties. Thus, this study demonstrates that catalyst-free PHU CANs made from well-designed multifunctional secondary amines can be easily synthesized and exhibit robust reprocessability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


