铜催化clok - wilson重排合成含四取代碳原子的二氢呋喃
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-26
DOI:10.1039/d5qo00401b
引用次数: 0
摘要
clok - wilson重排法是一种成熟的合成二氢呋喃的方法,但它主要导致二氢呋喃缺乏四取代的立体中心。在本研究中,我们引入了一种铜催化的Cloke-Wilson重排变体,该变体利用1,1,2,2-四取代环丙烷。这种方法允许合成具有四取代立体中心的多种二氢呋喃,产率从良好到优异不等。本文章由计算机程序翻译,如有差异,请以英文原文为准。
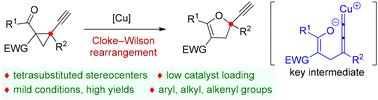
Copper-catalyzed Cloke–Wilson rearrangement for the synthesis of dihydrofurans containing tetrasubstituted carbon atoms†
The Cloke–Wilson rearrangement is a well-established method for synthesizing dihydrofurans, but it primarily results in dihydrofurans lacking tetrasubstituted carbon atoms. In this study, we introduce a copper-catalyzed variant of the Cloke–Wilson rearrangement that utilizes 1,1,2,2-tetrasubstituted cyclopropanes. This approach allows for the synthesis of a diverse range of dihydrofurans featuring tetrasubstituted carbon atoms, with yields ranging from good to excellent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


