稀土在千瓦级碱性海水电解槽中的腐蚀防护
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
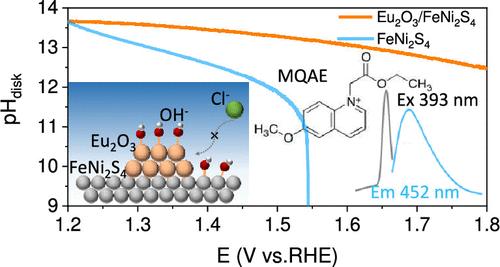
高电流密度下阳极OH -消耗对Cl -的竞争性吸附是制约碱性海水电解槽(ASWE)发展的重要因素。在此,我们提出了一种稀土腐蚀防护策略,该策略利用不参与反应的氧友好型稀土吸附OH -并维持ASWE中稳定的阳极催化的表面环境。采用差分电化学质谱法(dem)和电化学石英晶体微天平(EQCM)对传统镍网大电流阳极极板的氯腐蚀原因进行了分析。采用氯离子荧光探针、旋转环形圆盘电极(RRDE)和时间分辨吸收光谱标记n -乙氧羰基甲基-6-甲氧基喹啉溴化铵(MQAE)的原位荧光光谱对稀土的识别机理进行了研究。Eu2O3吸附OH -维持高电流pH环境,抑制Cl -吸附氧化,从而在500 mA cm-2电流密度下表现出超过1000小时的稳定性。此外,Eu2O3/FeNi2S4被组装成一个千瓦级ASWE,在17个腔室中,总面积为1081.5 cm2,并在80°C和30% KOH的工业条件下,在500 mA cm-2的电流密度下稳定运行超过100小时。技术经济分析(TEA)表明,稀土腐蚀防护策略可以提高ASWE的使用寿命,降低制氢成本,实现海水制氢的盈利,为解决ASWE的氯氧化腐蚀问题提供了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Corrosion Protection of Rare Earth for Kilowatt-Level Alkaline Seawater Electrolyzer
The competitive adsorption of Cl– caused by anode OH– consumption under high current density is an important factor restricting the development of an alkaline seawater electrolyzer (ASWE). Here, we propose a strategy for rare earth corrosion protection which utilizes oxygen friendly rare earths that do not participate in the reaction to adsorb OH– and maintain the surface environment for stable anodic catalysis in an ASWE. Differential electrochemical mass spectrometry (DEMS) and electrochemical quartz crystal microbalance (EQCM) were used to identify the causes of chlorine corrosion on the high current anode plate of traditional Ni mesh. In situ fluorescence spectra of N-ethoxycarbonylmethyl-6-methoxyquinolinium bromide (MQAE) labeled with a chloride ion fluorescence probe, a rotating ring disk electrode (RRDE), and a time-resolved absorption spectrum were used to test the recognition mechanism of rare earth. Eu2O3 adsorbs OH– to maintain a high current pH environment and inhibits Cl– adsorption oxidation, thereby exhibiting stability for over 1000 h at 500 mA cm–2 current density. Furthermore, Eu2O3/FeNi2S4 was assembled into a kilowatt-level ASWE in 17 chambers with a total area of 1081.5 cm2 and operated stably for over 100 h at a current density of 500 mA cm–2 under industrial conditions of 80 °C and 30% KOH. Technical economic analysis (TEA) indicates that the rare earth corrosion protection strategy can enhance the service life of ASWE and reduce the cost of hydrogen production for profitable seawater hydrogen production, providing a new approach to solve the chlorine oxidation corrosion problem in an ASWE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


