含多功能嵌段的双亲水嵌段共聚物的合成:由单一正阴离子共聚物自发形成多离子络合物胶束
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
通过多步合成得到了具有多官能团块的双亲水嵌段共聚物(DHBC)。首先是母体共聚物Par. Pol。采用RAFT聚合法制备了由聚低聚(乙二醇)-丙烯酸甲酯(P(OEGMEA))中性嵌段和聚丙烯酸(PAA)弱聚酸嵌段组成的P(OEGMEA)-b-PAA。然后,通过活化/酰胺化途径对PAA嵌段进行修饰,用N-(3-(二甲氨基)丙基)-N′-乙基碳二亚胺(EDC)和N-羟基琥珀酰亚胺(NHS)活化DHBC,得到活化共聚物Act。波尔。,然后与N-Boc乙二胺反应。由此产生的改性共聚物,命名为Ami。波尔。由P(OEGMEA)-b-P(AA-s-(酰基尿素)-s-(N-Boc))组成,在第二个区块上有几个官能团:来自PAA主链的丙烯酸酯,连接n -酰基尿素,最后连接N-Boc乙二胺。n -酰基尿素含有叔胺,而N-boc乙二胺添加了叔丁基保护的伯胺,这些伯胺随后可以用三氯乙酸(TCA)脱保护步骤去除,最终得到P(OEGMEA)-b-P(AA-s-(酰基尿素)-s-(AA/NH2))共聚物,标记为De. Pol。我们在改性的每个阶段(即亲本共聚物、活化共聚物、修饰共聚物和去保护共聚物)对DHBC进行了表征,使用NMR和元素分析相结合的方法来评估第二段中每个基团的单位数量。活化/酰胺化后,n -酰基脲基约占第二区块的13-32%,这取决于活化条件,而N-Boc乙二胺基的数量约为8-34%,这取决于酰胺化条件。我们证明了去保护后有效去除叔丁基保护基团而不损害DHBC。由于丙烯酸酯和胺功能的存在,活化,修饰和去保护共聚物表现出ph可调的自组装特性。利用动态光散射(DLS)、ζ电位测量和ATR-FTIR对pH值为2 ~ 9的样品进行了研究,发现pH值为4 ~ 9时,样品的胶束分布良好。结合测量,再加上DLS研究作为盐的功能,提供了胶束是由带正电的n -酰基尿素悬浮基和未修饰的带负电的丙烯酸酯物种之间的静电络合形成的证据。然后使用光和小角中子散射(SANS)的组合来表征胶束。值得注意的是,动态光散射(DLS)获得了5-7单种群胶束化的最佳pH范围。利用聚合物胶束模型成功拟合了SANS数据,该模型提供了胶束核半径R (pH = 5时改性共聚物为6.3±0.1 nm)、胶束壳中P(OEGMEA)链的旋转半径Rg(3.3±0.1 nm)和尺寸上的多分散性σ(13±1%)的信息。还研究了改性共聚物的SANS模式与浓度的关系,并通过添加硬球结构因子成功地拟合了数据,为胶束间相互作用提供了证据。最后,去保护共聚物的SANS模式显示核心半径减小(pH = 5时R = 5.3±0.1 nm),与去除体积较大的叔丁基一致。本文开发的方法允许dhbc的形成,由于添加了n -酰基尿素基团,dhbc不仅在水中表现出自组装特性,而且还呈现额外的官能团(在我们的情况下,伯胺)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
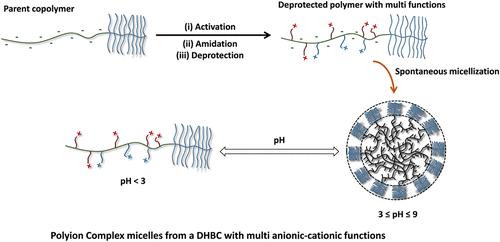
Synthesis of a Double-Hydrophilic Block Copolymer with a Multifunctional Block: Spontaneous Formation of Polyion Complex Micelles from a Single Cationic–Anionic Copolymer
A double-hydrophilic block copolymer (DHBC) exhibiting a multifunctional block was obtained via a multistep synthesis. First, the parent copolymer, Par. Pol., P(OEGMEA)-b-PAA, composed of a neutral block of poly(oligo(ethylene glycol))-methyl ether acrylate (P(OEGMEA)) and a weak polyacid block of poly(acrylic acid) (PAA), was synthesized by RAFT polymerization. Then, the PAA block was modified via the activation/amidation route, using N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS) to activate the DHBC, yielding the activated copolymer Act. Pol., before reaction with N-Boc ethylenediamine. The resulting amidated copolymer, named Ami. Pol., composed of P(OEGMEA)-b-P(AA-s-(Acyl urea)-s-(N-Boc)), exhibits several functional groups on the second block: acrylates from the PAA backbone, pending N-Acyl urea, and finally pending N-Boc ethylenediamine. N-Acyl urea exhibits tertiary amines, while N-boc ethylenediamine adds primary amines protected by a tert-butyl group, which can later be removed by a deprotection step using trichloroacetic acid (TCA), yielding the final P(OEGMEA)-b-P(AA-s-(Acyl urea)-s-(AA/NH2)) copolymer, labeled De. Pol. We characterized the DHBC at every stage of the modification (i.e., parent copolymer, activated copolymer, amidated copolymer, and deprotected copolymer) using a combination of NMR and elemental analysis to assess the number of units of each group in the second block. After activation/amidation, N-Acyl urea groups represent ca. 13–32% of the second block, depending on the activation conditions, while the amount of N-Boc ethylenediamine groups is ca. 8–34%, depending on the amidation conditions. We demonstrated the efficient removal of the tert-butyl protection groups after deprotection without any damage to the DHBC. Due to the presence of acrylates and amine functions, the activated, amidated, and deprotected copolymers exhibit pH-tunable self-assembling properties. Samples were studied at pH values ranging from 2 to 9, using dynamic light scattering (DLS), ζ-potential measurements, and ATR-FTIR, and well-defined micelles were observed at pH values ranging from 4–9. The combination of measurements, coupled with DLS studies as a function of salt, provided evidence that micelles were formed by electrostatic complexation between the positively charged N-Acyl urea pending groups and the unmodified negatively charged acrylate species. Micelles were then characterized using a combination of light and small-angle neutron scattering (SANS). Notably, an optimum pH range for micellization of 5–7 with a single population was obtained by dynamic light scattering (DLS). SANS data were successfully fitted using a model of polymer micelles, which provided information about the core radius of the micelles R (6.3 ± 0.1 nm for amidated copolymer at pH = 5), the gyration radius of the P(OEGMEA) chains in the micelle shell Rg (3.3 ± 0.1 nm), and the polydispersity in size σ (13 ± 1%). SANS patterns of amidated copolymers as a function of concentration were also studied, and data were successfully fitted by adding a hard-sphere structure factor, providing evidence of intermicellar interactions. Finally, SANS patterns of the deprotected copolymer showed a decrease in the core radius (R = 5.3 ± 0.1 nm at pH = 5), consistent with the removal of the bulky tert-butyl groups. The method developed here allows the formation of DHBCs that not only exhibit self-assembling properties in water due to the addition of N-Acyl urea groups but also present extra functional groups (in our case, primary amines).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


