氧化还原循环中Au(111)单晶电极表面的各向异性粗化
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用电化学扫描隧道显微镜(EC-STM)研究了在0.1 M高氯酸(HClO4)溶液中氧化还原循环(ORCs)过程中Au(111)单晶电极表面的不均匀粗化。结果表明,即使在超纯HClO4中,少量杂质的存在也会导致同一晶体上三种可区分的表面演变:由吸附原子和空位岛形成的表面粗糙化,金的溶解导致空位岛形成(与阶梯线退化一起),以及即使在氧化还原循环过程中表面仍保持完整。通过在HClO4溶液中加入10 μM的H2SO4和HCl,研究了微量杂质(特别是硫酸盐(SO42 -)和氯化物(Cl -))对表面结构发展的影响。结果表明,硫酸盐对均匀粗化有显著促进作用,而氯化物则加速了金的溶解和阶梯线还原。这些发现强调了少量杂质在改变金表面电化学行为中的关键作用,以及表面结构的局部演变对这些影响的敏感性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
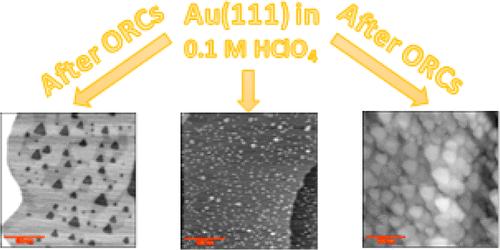
Anisotropic Roughening of a Au(111) Single-Crystal Electrode Surface in HClO4 Solution during Oxidation–Reduction Cycles
This study investigates the inhomogeneous roughening of a Au(111) single-crystal electrode surface during oxidation–reduction cycles (ORCs) in a 0.1 M perchloric acid (HClO4) solution using electrochemical scanning tunneling microscopy (EC-STM). The results reveal that, even in ultrapure HClO4, the presence of minor impurities can lead to three distinguishable surface evolutions, on one and the same crystal: surface roughening by the formation of adatom and vacancy islands, gold dissolution resulting in vacancy island formation (in conjunction with step-line recession), and the surface remaining intact even during oxidation–reduction cycling. The impact of trace impurities, specifically sulfate (SO42–) and chloride (Cl–), on the surface structure development is investigated by adding 10 μM of H2SO4 and HCl to the HClO4 solution. Our results reveal that sulfate significantly promotes uniform roughening, while chloride accelerates gold dissolution and step-line recession. These findings highlight the critical role of minor impurities in altering the electrochemical behavior of gold surfaces and how sensitive the local evolution of the surface structure is to these effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


