由市售二胺衍生的新型HAT有机催化剂的设计与合成应用
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-26
DOI:10.1039/d5qo00509d
引用次数: 0
摘要
以市售的二胺类化合物为原料制备了一系列的氢原子转移(HAT)有机催化剂,并研究了它们在光诱导HAT催化方面的应用。这些易于获得的HAT有机催化剂与Fukuzumi光氧化还原催化剂的组合可以实现从简单碳氢化合物到复杂分子的各种功能化底物的高效和位点选择性的C - H烷基化。多功能分子的位点选择性C-H烷基化也得到了证实。值得注意的是,双功能底物的连续一锅光诱导双烷基化可以实现。机理研究表明,二胺化合物的一个氮原子上的1-萘基甲基在反应中起着至关重要的作用,它可以诱导二胺的另一个氮原子上的阳离子胺自由基的生成,作为HAT过程的活性中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
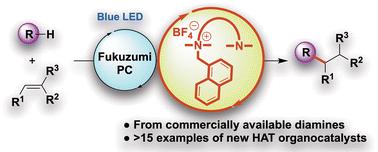
Design and synthetic utility of new HAT organocatalysts derived from commercially available diamines†‡
A series of hydrogen-atom transfer (HAT) organocatalysts were conveniently prepared from commercially available diamine compounds, and their utility in photoinduced HAT catalysis ability was investigated. The combination of these readily available HAT organocatalysts with the Fukuzumi photoredox catalyst enables efficient and site-selective C–H alkylation of various functionalized substrates ranging from simple hydrocarbons to complex molecules. Notably, the sequential one-pot photoinduced dialkylations of bifunctional substrates can be realized. Mechanistic studies suggested that the 1-naphthylmethyl moiety on one nitrogen atom of the diamine compounds plays a crucial role in the reaction by inducing the facile generation of a cationic aminium radical on the other nitrogen of the diamine as an active intermediate for the HAT process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


