DNA甲基转移酶1通过桥接m5C RNA甲基化调节线粒体功能
IF 16.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
DNA甲基转移酶1 (DNMT1)是一种已知的DNA甲基化维持酶。其复制焦点靶向序列(RFTS)结构域的点突变可导致晚发性神经变性,如常染色体显性小脑性共济失调-耳聋和嗜睡症(ADCA-DN)障碍。在这里,我们证明DNMT1具有结合mRNA转录本的能力,并通过募集NOP2/Sun RNA甲基转移酶2 (NSUN2)促进5-甲基胞嘧啶(m5C) RNA甲基化。反过来,RNA m5C甲基化促进了那些调节线粒体功能的基因的RNA稳定性。当DNMT1 RFTS结构域在小鼠中发生突变时,它会引发DNMT1-RNA的异常相互作用,并显著提高部分代谢基因的m5C RNA甲基化和RNA稳定性。因此,代谢RNA转录物水平升高导致累积氧化应激、线粒体功能障碍和神经系统症状。总之,我们的研究结果揭示了DNMT1在调节DNA和RNA甲基化中的双重作用,这进一步调节了线粒体功能,揭示了DNMT1突变诱导的神经变性的致病机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
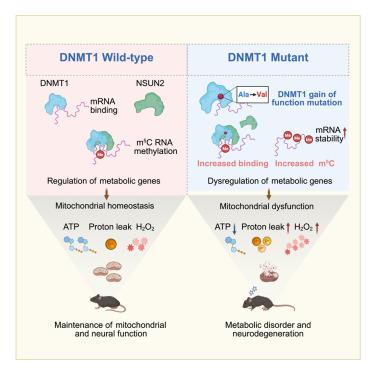
DNA methyltransferase 1 modulates mitochondrial function through bridging m5C RNA methylation
DNA methyltransferase 1 (DNMT1) is an enzyme known for DNA methylation maintenance. Point mutations in its replication focus targeting sequence (RFTS) domain lead to late-onset neurodegeneration, such as autosomal dominant cerebellar ataxia-deafness and narcolepsy (ADCA-DN) disorder. Here, we demonstrated that DNMT1 has the capability to bind to mRNA transcripts and facilitate 5-methylcytosine (m5C) RNA methylation by recruiting NOP2/Sun RNA methyltransferase 2 (NSUN2). RNA m5C methylation, in turn, promotes RNA stability for those genes modulating mitochondrial function. When the DNMT1 RFTS domain is mutated in mice, it triggers aberrant DNMT1-RNA interaction and significantly elevated m5C RNA methylation and RNA stability for a portion of metabolic genes. Consequently, increased levels of metabolic RNA transcripts contribute to cumulative oxidative stress, mitochondrial dysfunction, and neurological symptoms. Collectively, our results reveal a dual role of DNMT1 in regulating both DNA and RNA methylation, which further modulates mitochondrial function, shedding light on the pathogenic mechanism of DNMT1 mutation-induced neurodegeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


