胞浆内NADK对叶酸依赖的核苷酸合成是有条件必需的
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
烟酰胺腺嘌呤二核苷酸激酶(Nicotinamide adenine dinucleotide kinase, NADK)催化NAD+磷酸化产生NAD磷酸,这是NADPH的氧化形式,在驱动还原性代谢中起关键作用的辅助因子。癌细胞共表达两种不同的NAD激酶,它们的定位不同(NADK,细胞质;NADK2线粒体)。在数百种癌细胞系中进行的CRISPR筛选表明,这两种细胞在传统培养基中生长是必不可少的。相反,在人血浆样培养基中,NADK缺失会损害细胞生长。在这里,我们将这种条件依赖性追溯到叶酸的可用性。NADPH是二氢叶酸还原酶(DHFR)的首选辅助因子,该酶介导叶酸的代谢激活。我们发现,在低叶酸条件下,NADK是使细胞内nadph驱动的DHFR活性足以维持叶酸依赖的核苷酸合成所必需的。我们的研究结果揭示了条件NADK必要性的基础,并表明叶酸的可用性决定了DHFR活性是否可以由NADH等替代电子供体维持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
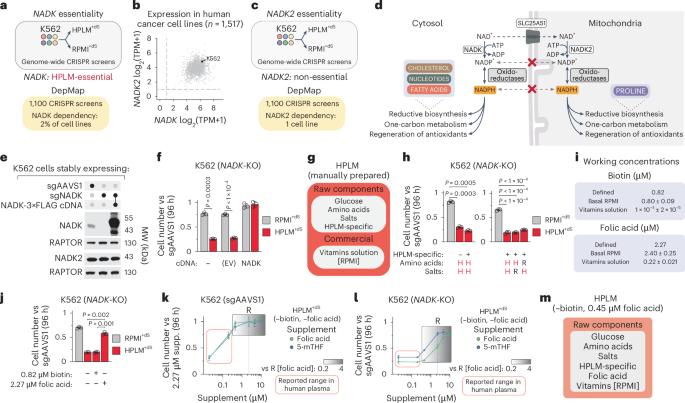

Cytosolic NADK is conditionally essential for folate-dependent nucleotide synthesis
Nicotinamide adenine dinucleotide kinase (NADK) catalyses the phosphorylation of NAD+ to produce NAD phosphate, the oxidized form of NADPH, a cofactor that serves a critical role in driving reductive metabolism. Cancer cells co-express two distinct NAD kinases that differ by localization (NADK, cytosol; NADK2, mitochondria). CRISPR screens performed across hundreds of cancer cell lines indicate that both are dispensable for growth in conventional culture media. By contrast, NADK deletion impaired cell growth in human plasma-like medium. Here we trace this conditional NADK dependence to the availability of folic acid. NADPH is the preferred cofactor of dihydrofolate reductase (DHFR), the enzyme that mediates metabolic activation of folic acid. We find that NADK is required for enabling cytosolic NADPH-driven DHFR activity sufficient to maintain folate-dependent nucleotide synthesis under low folic acid conditions. Our results reveal a basis for conditional NADK essentiality and suggest that folate availability determines whether DHFR activity can be sustained by alternative electron donors such as NADH. In this study, Flickinger et al. uncover the essential role of cytosolic NADK and why the relative importance of this role further depends on folate availability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


