两步法合成密集功能化n -桥接八氢苯并呋喃和六氢苯并呋喃
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了用两步法合成密集功能化n桥八氢苯并呋喃和六氢苯并呋喃的新工艺。其机理包括一种新的催化重排反应、Diels-Alder反应和分子内氮杂加成环化反应。初步生物实验表明,化合物3d对金黄色葡萄球菌有较强的抑制作用,而化合物4d对斜纹夜蛾有较好的杀虫活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
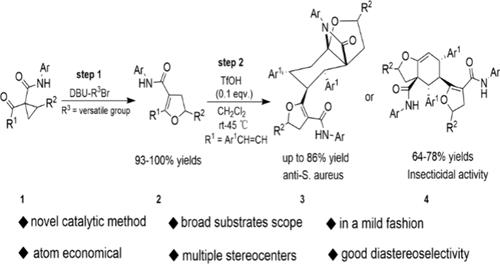
A Two-Step Process for the Synthesis of Densely Functionalized N-Bridged Octahydrobenzofurans and Hexahydrobenzofurans
A new process for the synthesis of densely functionalized N-bridged octahydrobenzofurans and hexahydrobenzofurans, by means of a two-step protocol, is reported. The mechanism involves a novel catalytic rearrangement reaction, a Diels–Alder reaction, and an intramolecular aza-addition cyclization. Preliminary bioassays showed that compound 3d more strongly inhibited Staphylococcus aureus, while compound 4d displayed excellent insecticidal activity against Spodoptera litura Fabricius.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


