甲烷氧化偶联中La2O2CO3结构敏感性的理论研究及其与La2O3的比较
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
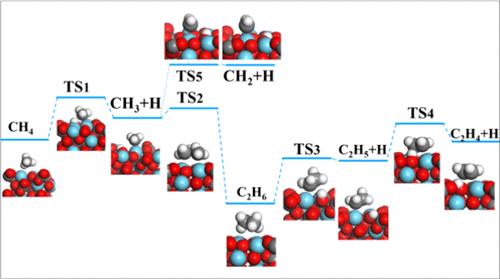
通过甲烷氧化偶联(OCM)将甲烷转化为C2+烃产物可以更有效地提高甲烷的利用价值,以往的研究多集中在La2O3上,而关于衍生催化剂La2O2CO3的报道较少。因此,本工作旨在深入探索La2O2CO3在OCM反应中的结构敏感性,并比较其与La2O3的催化性能。本文采用DFT计算和微动力学模拟研究了La2O3(110)、La2O2CO3(100)和La2O2CO3(110)表面的反应机理,探讨了不同晶面对OCM反应的催化活性和C2+选择性。计算结果表明,基于CH3·分解反应和CH3·偶联反应的活化能差异,La2O2CO3催化剂对C2+的选择性高于La2O3催化剂。微动力学模拟表明,甲烷氧化分解是整个OCM反应过程的主要限速步骤,La2O2CO3(110)表面比La2O2CO3(100)表面更有利于C2+物质的形成。希望本研究的发现能够加深研究者对OCM反应机理的认识,为开发更高效、选择性更强的催化剂提供重要的理论指导和实践参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Theoretical Study on the Structure Sensitivity of La2O2CO3 in Oxidative Coupling of Methane and Its Comparison with La2O3
Converting methane into C2+ hydrocarbon products through oxidative coupling of methane (OCM) can more effectively enhance the utilization value of methane, and previous studies are usually focused on La2O3, but there are few reports on the derivative catalyst La2O2CO3. Therefore, this work aims to deeply explore the structural sensitivity of La2O2CO3 in the OCM reaction and compare its catalytic performance with that of La2O3. In this article, we used DFT calculations and microkinetic simulations to investigate the reaction mechanisms on the surfaces of La2O3(110), La2O2CO3(100), and La2O2CO3(110), and explored the catalytic activity and C2+ species selectivity of different crystal planes for the OCM reaction. The calculation results show that the La2O2CO3 catalyst with higher selectivity for C2+ species compared to the La2O3 catalyst, based on the difference in activation energy between the CH3· decomposition reaction and the CH3· coupling reaction. Microkinetic simulations reveal that methane oxidative decomposition is the main rate-limiting step in the entire OCM reaction process, and the La2O2CO3 (110) surface is more favorable for the formation of C2+ species than that of La2O2CO3 (100). It is hoped that the findings of this study can deepen researchers’ understanding of the OCM reaction mechanism and provide important theoretical guidance and practical reference for the development of more efficient and selective catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


