利用工程希瓦氏菌生物电合成直接将二氧化碳转化为苹果酸盐
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
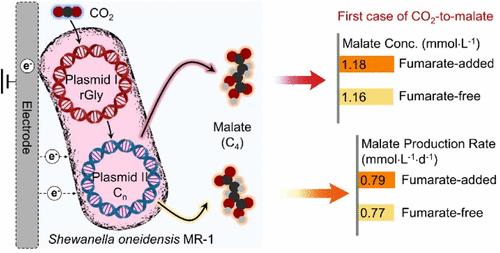
微生物电合成(MES)为二氧化碳的增殖提供了一种可持续和低碳的方法,其中希瓦氏菌(S. oneidensis) MR-1被认为是MES的理想微生物。然而,由于s.o oneidensis MR-1无法执行细胞内甲酸盐同化途径,因此没有研究表明其可以直接将CO2代谢成多碳(C2+)产物。在这里,我们提供了直接生物电化学二氧化碳还原到苹果酸的C4产品的初步概念证明证据。其中,二氧化碳转化为苹果酸盐的产物浓度达到了1.18 mmol·L-1,这标志着第一次直接生物电合成C4化合物。这种显著的二氧化碳到c4转化性能归功于双质粒系统的成功实施,该系统促进了还原甘氨酸途径(质粒I)的过度表达,以吸收二氧化碳衍生的甲酸,以及替代苹果酸生物合成途径(质粒II),以引导代谢中间体进行苹果酸的生物合成。推进二氧化碳向负碳C2+生物产品的增值,我们在微生物中设计的复杂双质粒系统可以进一步改进,以实现可扩展的二氧化碳生物电解,目的是促进工业应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Direct CO2 Transformation to Malate via Bioelectrosynthesis upon Engineered Shewanella oneidensis
Microbial electrosynthesis (MES) offers a sustainable and low-carbon approach for CO2 valorization, with Shewanella oneidensis (S. oneidensis) MR-1 identified as an ideal microbe for MES. However, no prior research has demonstrated that S. oneidensis MR-1 can directly metabolize CO2 into multicarbon (C2+) products due to its inability to perform the intracellular formate assimilation pathway. Here, we provide initial proof-of-concept evidence of direct bioelectrochemical CO2 reduction to the C4 product of malate. Specifically, the transformation of CO2 to malate attains a notable production concentration of 1.18 mmol·L–1, marking the first instance of direct C4 compound bioelectrosynthesis. Such remarkable CO2-to-C4 conversion performances are attributed to the successful implementation of dual-plasmid systems in S. oneidensis MR-1, which facilitate the overexpression of the reductive glycine pathway (Plasmid I) for assimilating CO2-derived formate and the alternative malate biosynthetic pathway (Plasmid II) to channel metabolic intermediates toward the biosynthesis of malate. Advancing CO2 valorization toward carbon-negative C2+ bioproducts, our sophisticated dual-plasmid systems engineered in microbes can be further refined for scalable CO2 bioelectrolysis with the objective of facilitating industrial applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


