含烯基取代的四氯代立体中心的氧吲哚-吡唑酮缀合物的对映选择性和非对映发散性构造
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00499c
引用次数: 0
摘要
氯化立体碳中心是医药试剂和合成中间体中的重要元素。本文报道了一种以氧吲哚和吡唑酮为药效团的烯基取代季氯代立体中心的构建方法。值得注意的是,通过适当的碱和溶剂的组合,双键的构型是可以改变的。此外,以天然奎尼丁为催化剂,实现了z型产物的对映选择性合成,使氯化产物具有良好的产率和立体选择性。初步研究了1H-NMR滴定法,以深入了解双键构型的控制。此外,对这些新合成的化学实体进行了抗肿瘤活性评价,发现产物(E)-5ca是一种很有前景的抗肿瘤试剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
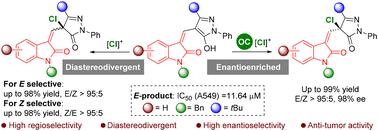
Enantioselective and diastereodivergent construction of oxindole–pyrazolone conjugates bearing an alkenyl substituted quaternary chlorinated stereogenic centre†
Chlorinated stereogenic carbon centres are important elements both in pharmaceutical reagents and synthetic intermediates. Herein, a novel methodology is reported for the construction of a rarely developed alkenyl substituted quaternary chlorinated stereogenic centre, featured in oxindole and pyrazolone pharmacophores. Remarkably, the configuration of the double bond was switchable via the combination of a suitable base and solvent. In addition, the enantioselective synthesis of Z-type products was achieved with natural quinidine as a catalyst, affording the chlorinated products in excellent yields and stereoselectivities. Preliminary 1H-NMR titration was studied to gain insights into the control of the double bond's configuration. Moreover, the anti-tumour activity against the A549 cell-line of these newly synthesized chemical entities was evaluated, and the product (E)- was revealed to be a promising anti-tumour agent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


