铜催化芳基邻碳氢硫代醛的瞬态定向基团策略
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00472a
引用次数: 0
摘要
过渡金属催化C-H功能化是合成芳基硫化物的一种有效方法。目前的反应主要依赖于使用预装的定向组,这限制了它们的实际应用。在这里,我们报告了第一个瞬态定向基团使能的C-H硫基化的例子。以氨基苯甲酸为催化剂,芳基醛形成瞬时亚胺导向基团,并进行铜催化芳基邻羟基硫代化。该反应具有广泛的底物范围,便于获得各种芳基硫化物。此外,这些反应的合成效用已经通过它们在与药物和生物活性分子合成相关的关键中间体中的应用得到了证明。本文章由计算机程序翻译,如有差异,请以英文原文为准。
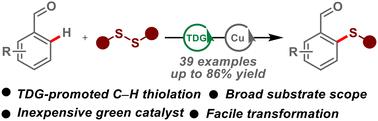

Copper-catalyzed aryl ortho-C–H thiolation of aldehydes via a transient directing group strategy†
Transition metal-catalyzed C–H functionalization represents a robust method for the synthesis of aryl sulfides. The current reactions primarily rely on the use of preinstalled directing groups, which limits their practical applications. Herein, we report the first example of transient directing group-enabled C–H thiolation. Using an aminobenzoic acid as catalyst, aryl aldehydes form the transient imine directing groups and undergo copper-catalyzed aryl ortho-C–H thiolation. The reactions feature a broad substrate scope, facilitating easy access to a diverse range of aryl sulfides. Furthermore, the synthetic utilities of these reactions have been demonstrated by their applications to key intermdediates relevant to the synthesis of drug and bioactive molecule.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


