四苯基乙烯或萘酰亚胺功能化的树突状咔唑原:自组装可视化,三种不同的力触发荧光响应,以及先进的防伪应用
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00393h
引用次数: 0
摘要
设计合成了3个1,8-萘酰亚胺修饰的供体-π-受体型和3个四苯基乙烯功能化的荧光树突状咔唑衍生物。这六种树状咔唑类化合物是典型的聚集诱导发射(AIE)发光物质。特别地,含有三聚咔唑和两个1,8-萘酰亚胺基团的aie活性3CzB2Nap具有聚集触发自组装的特点,并通过扫描电镜技术成功地实现了其有趣的自组装过程的可视化。值得注意的是,从六种制备的树突状咔唑aigens中观察到三种不同类型的各向异性机械力诱导荧光响应。更具体地说,7CzB2TPE和3CzB2Nap在研磨后没有荧光变化;1CzB2TPE和3CzB2TPE显示不可逆的机械荧光现象;1CzB2Nap和7CzB2Nap表现出可逆的机械荧光特性。为了深入阐明六种枝晶AIEgens、粉末和单晶的三种不同类型的力响应发射特征,进行了差示扫描量热实验和研磨前后分子堆积结构的理论模拟计算。基于观察到的三种不同的力依赖荧光现象,精心构建了两种先进的信息安全系统,包括多层防伪和多模式信息加密。本文章由计算机程序翻译,如有差异,请以英文原文为准。
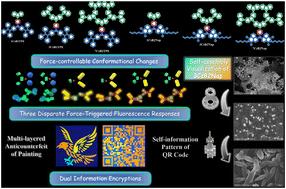
Tetraphenylethylene or naphthalimide-functionalized dendritic carbazole AIEgens: self-assembly visualization, three disparate force-triggered fluorescence responses, and advanced anticounterfeiting applications†
Three 1,8-naphthalimide-modified donor–π–acceptor-type and three tetraphenylethylene-functionalized fluorogenic dendritic carbazole derivatives are designed and synthesized. These six dendrimer-like carbazole-containing compounds are typical aggregation-induced emission (AIE) luminogens. Specially, the AIE-active possessing trimeric carbazole and two 1,8-naphthalimide groups features aggregation-triggered self-assembly, and the visualization of its intriguing self-assembly process is successfully realized through scanning electron microscopy technology. Notably, three types of contrasting anisotropic mechanical force-induced fluorescence responses from the six prepared dendritic carbazole AIEgens are observed. More specifically, and do not exhibit fluorescence changes after grinding; and display irreversible mechanofluorochromic phenomena; and show reversible mechanofluorochromic characteristics. To deeply elucidate the three distinct types of force-responsive emissive features of the six dendritic AIEgens, powder and single-crystal X-ray diffraction, differential scanning calorimetry experiments, and theoretical simulation calculations of molecular packing structures before and after grinding are carried out. Based on the observed three disparate force-dependent fluorescence phenomena, two advanced information security systems involving multilevel painting anticounterfeiting and multimode information encryption are elaborately constructed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


