pd催化三氟甲基苯并恶唑酮与2-甲基三亚甲基碳酸酯扩环反应合成八元三氟甲基苯并恶唑酮及其机理研究
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d4qo02076f
引用次数: 0
摘要
报道了一种pd催化的三氟甲基苯并恶嗪酮与2-甲基二甲基碳酸三亚甲基的扩环反应。该方法具有高效率和广泛的官能团耐受性,可快速组装出一系列有价值的8元三氟甲基苯并恶唑嗪,且收率高。通过产品的简单变换,证明了该方法的综合实用性。最后,通过密度泛函理论(DFT)计算对反应机理进行了研究,结果表明,在苯并恶嗪酮脱羧重建环过程中,羰基对位点选择性的影响大于三氟甲基。本文章由计算机程序翻译,如有差异,请以英文原文为准。
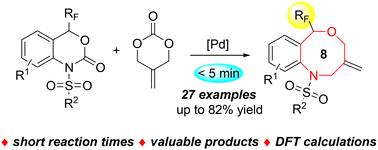
Synthesis of 8-membered trifluoromethyl benzoxazocines via a Pd-catalyzed ring-expansion reaction of trifluoromethyl benzoxazinones with 2-methylidenetrimethylene carbonate and mechanistic investigations†
We report a Pd-catalyzed ring-expansion reaction of trifluoromethyl benzoxazinones with 2-methylidenetrimethylene carbonate. This method exhibits high efficiency and broad functional group tolerance, which leads to the rapid assembly of a series of valuable 8-membered trifluoromethyl benzoxazocines with good yields in a simple manner. The synthetic utility of the approach is demonstrated by the facile transformation of product. The reaction mechanism was also investigated by density functional theory (DFT) calculations, suggesting that the carbonyl group has a greater influence on the site-selectivity than the trifluoromethyl group in the decarboxylative ring re-construction process of benzoxazinones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


