IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从尖晶石宝石(MgAl2O4)到层状双氢氧化物,自然界长期以来一直依赖于电荷互补金属离子(如二价金属离子(M2+)和Al3+)之间的组合来创造各种有价值的材料。然而,对于金属有机框架(mof),异质金属组合如Mg-Al明显不存在。在这里,我们报道了在合成含有M2+/Al3+三聚簇(M = Mg, Mn, Co, Ni)的异质金属al - mof方面的突破。M之间的协同效应(2)氯化物和铝乳酸中起关键作用的合作结晶M2 +和与pore-space-partitioned财政部(acs拓扑分区)与快速结晶动力学(约3小时)。新M2 + /与mof表现出高度可调的孔隙度和相当高的吸收二氧化碳和小分子烃类(112立方厘米/ g二氧化碳,乙炔,176立方厘米/ g 156立方厘米/ g C2H4 C2H6和163立方厘米/ g)在298 K和1条。高吸收率和高选择性(C2H2/CO2可达8.5,C2H2/C2H4可达10.8)使得C2H2/CO2或C2H2/C2H4混合气体的有效分离得到了实验突破性实验的证实。本文章由计算机程序翻译,如有差异,请以英文原文为准。
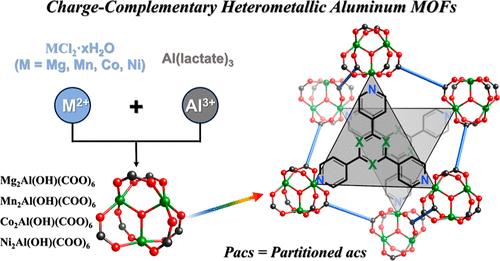
Heterometallic Aluminum Metal–Organic Frameworks
From spinel gemstone (MgAl2O4) to layered double hydroxides, nature has long relied on combinations between charge-complementary metal ions such as divalent metal ions (M2+) and Al3+ to create diverse valuable materials. However, for metal–organic frameworks (MOFs), heterometallic combinations such as Mg–Al are conspicuously absent. Here, we report a breakthrough in the synthesis of heterometallic Al-MOFs containing M2+/Al3+ trimeric clusters (M = Mg, Mn, Co, Ni). The synergistic effect between M(II) chlorides and aluminum lactate plays a critical role in the cooperative crystallization of M2+ and Al3+ into pore-space-partitioned MOFs (partitioned acs topology) with fast crystallization kinetics (about 3 h). New M2+/Al3+ MOFs exhibit highly tunable porosity and extraordinarily high uptakes for CO2 and small hydrocarbon molecules (112 cm3/g for CO2, 176 cm3/g for C2H2, 156 cm3/g for C2H4, and 163 cm3/g for C2H6) at 298 K and 1 bar. The high uptake capacity coupled with high selectivity (up to 8.5 for C2H2/CO2, 10.8 for C2H2/C2H4) gives rise to efficient separations of either C2H2/CO2 or C2H2/C2H4 gas mixtures, as confirmed by experimental breakthrough experiments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


